Chem 334 - Summer 1998 - Organic Chemistry I
1. (20 pts) Write complete IUPAC names for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 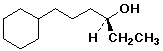
b) 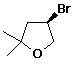
c) 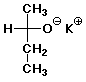
d) 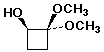
e) 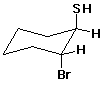
2. (15 pts) Complete each of the following acid-base reactions. Use the tables of pKa data to determine whether the equilibrium will be more favorable to the right or to the left.
a) ![]()
b) ![]()
c) ![]()
d) ![]()
e) What would be the major form (ionized or neutral) of methanol in aqueous solution at
pH 1, pH 7, pH 14 ?
3. (15 pts) Arrange the following in order of reactivity by writing "MOST" and "LEAST" next to the most and least reactive (or highest and lowest values of the property, respectively).
a) acidity
1-propanol / / 2-propanol / / 2-methyl-2-propanol
b) nucleophilicity
NaSH / / NaOEt / / EtOH
c) ease of dehydration by sulfuric acid at 130°
1-pentanol / / cyclopentanol / / tert-butyl alcohol
d) substitution/elimination ratio from NaOEt in EtOH
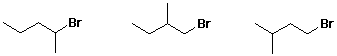
e) rate of reaction between NaOH and CH3I
in the gas phase / / in water / / in acetone
4. (15 pts) Complete the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final product(s). Indicate stereochemistry when it might be expected to be specific.
a) 
b) ![]()
c) ![]()
d) 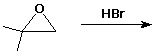
e) ![]()
5. (15 pts) Devise a synthetic sequence for the molecule shown below, starting with any alcohols having four or fewer carbons plus any needed inorganic reagents or solvents..
6. (10 pts) Write a complete mechanism that shows what would happen in the following reaction. If the starting compound is optically active, then the product is optically active. Show all steps.
7. (10 pts) Write a complete mechanism that shows how the following reaction occurs. Show all steps.