Organic Chemistry I
Dr. Carl C. Wamser
1. (15 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 
3-bromo-6-cyclobutyl-6-methylnonane
b) 
8,8-dimethyl-2-propylbicyclo[3.2.1]octane
c) 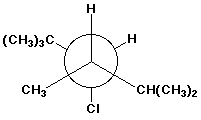
3-chloro-2,2,4,5-tetramethylhexane
2. (15 points) Write clear structures that show the following:
a) the best Lewis structure for HCNO (bonded in that order)
b) the Lewis acid-base reaction between BF3 and CH3OH
c) a compound with five carbons, all of which are sp2 hybridized
lots of possibilities, such as
d) all possible isomers with the name dimethylcyclopropane
e) the axial conformation of bromocyclohexane
3. (10 points) Examine the compound below and answer the
following questions about it.
a) (2 points) What is its molecular formula?
C5H7Br
b) (2 points) How many carbon atoms are sp3-hybridized?
all five
c) (2 points) How many primary hydrogens are there?
the 3 on the methyl group
d) (2 points) How many C-C bonds are there?
6
e) (2 points) Write a structure for a stereoisomer.
4. (15 points) Arrange each of the following in order with respect to the particular property indicated. Write MOST under the compound with the highest value or largest property and LEAST under the compound with the lowest value or smallest property.
a) boiling point

b) basicity
![]()
c) relative amount of axial chloro at equilibrium

d) relative amount of conjugate base at pH 7
![]()
e) acidity

5. a) (5 points) Create a favorable acid-base reaction (left to right) using ammonia, ethanol, and their conjugate bases. Indicate the pKa values you used to decide on the favorability.
![]()
b) (5 points) Complete a balanced acid-base reaction from the reactants shown below, identify the acid and base on each side of the equation, and determine whether the equilibrium will be favored to the right or to the left.

6. (15 points) Write Newman projections for all three staggered conformations of 2,2-dimethylpentane, looking down the C3-C4 bond. Select the most stable conformation.
2,2-dimethylpentane
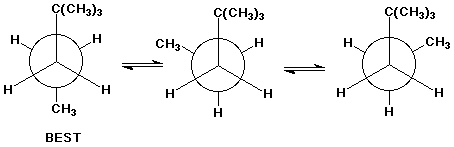
Also write one Newman diagram looking down the C2-C3 bond. Explain why the view down the C3-C4 bond is more informative in identifying the best conformation.
All positions on C2 are the same, so there are no distinctive conformations to choose from.
7. (20 points) Write both chair conformations for both the trans and cis isomers of 4-methylcyclohexanol, shown in a flat-ring structure below. The chair forms are started for you and are labeled A , B , C , D .
![]()
trans-4-methylcyclohexanol:
cis-4-methylcyclohexanol:
Select the more stable conformation for each compound.
A and C
Among the four conformations, select the most stable.
A
The Newman diagram below should be equivalent to one of your conformations. Indicate which one and write the Newman diagram for the other conformation of that compound.

this is C