
Chapter 9 - Alcohols and Thiols

Functional groups
alcohol: R-O-H (hydroxyl group)
thiol: R-S-H (sulfhydryl group)
Alcohol Classification
- based on the carbon the OH group is attached to:
1° , 2° , 3°
- methyl alcohol
CH3OH
- ethyl alcohol
CH3CH2OH (1°)
- isopropyl alcohol
(CH3)2CHOH (2°)
- t-butyl alcohol
(CH3)3COH (3°)
Alcohol Nomenclature
- OH group takes priority over -ene
- it must be in the parent chain
- the direction of numbering gives it the lower possible number
- -ol suffix with number designation
- name other substituents and multiple bonds as usual
- more than one alcohol named as a -diol, -triol, etc.
Alcohol Examples
- common names for alcohols:
alkyl alcohol
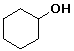
cyclohexyl alcohol or cyclohexanol

trans-4-methylcyclohexanol
Alcohol Example
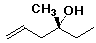
(R)-3-methyl-5-hexen-3-ol
Thiol Nomenclature
- the parent is the longest chain that contains the -SH group
- the IUPAC suffix is -thiol
- Common names: name the alkyl group bonded to sulfur followed
by the word mercaptan
example: 2-butanethiol or sec-butyl mercaptan
Structure of Alcohols
- sp3 O with two covalent bonds and two lone pairs
- The bond angle about oxygen is about 105°,
explained as greater repulsions of the lone pairs
towards the bonding pairs
Structure of Thiols
- The bond angle about sulfur in methanethiol is 100.3°,
explained as greater p character in the bonding orbitals
Properties of Alcohols
- Alcohols are polar compounds due to the polar C-O and O-H
bonds
- They interact with themselves and with other polar compounds
by dipole-dipole interactions
- Dipole-dipole interaction:
the attraction between the positive end of one dipole and the
negative end of another
Hydrogen Bonding
- attraction between the positive end of one dipole (an H bonded
to F, O, or N - atoms of high electronegativity) and the negative
end of a dipole, usually a lone pair on F, O, or N
- in alcohols, O lone pairs interact with polar H bonds
- covalent O-H bond strength ~ 100 kcal/mole
- O...H (H-bond) strength ~ 5 kcal/mole
Effects of H-Bonding
- alcohols have higher boiling points than alkanes (nonpolar)
or alkyl halides (polar, but no H-bonds)
- ethers are polar but have no H-bonds
(pentane and diethyl ether both boil at about 35°, but 1-butanol
has a bp of 117°)
- H-bonds hold together the strands of DNA ("Velcro"
effect)
- H-bonding increases water solubility
Properties of Thiols
- The S-H bond is only slightly polar
- Thiols show little association by hydrogen bonding
- Thiols have lower boiling points and are less soluble in
water than alcohols
Acid-Base Reactions
- remember analogy with water
- reactions as bases:
H2O + H+ <==> H3O+
ROH + H+ <==> ROH2+ (an oxonium
ion)
- reactions as acids:
H2O + B- <==> B-H + OH-
ROH + B- <==> B-H + RO- (an alkoxide
ion)
Acidity of Alcohols
- alcohols about as acidic as water
MeOH more acidic, EtOH less acidic
3° alcohols much weaker acids
- pKa values: 3° > 2° > 1° > MeOH
18 , 17, 16, 15.5 (compare H2O: pKa =
15.7)
- tBuOH + NaOH ---> unfavorable
Alkoxide Anions
- deprotonation of alcohols gives alkoxide anions
CH3OH + NaNH2 --->
NH3 + CH3O-
Na+ (sodium methoxide)
- most commonly made by direct reaction with active metals
CH3OH + Na ---> 1/2 H2
+ CH3O- Na+
(CH3)3COH + K --->
1/2 H2 + (CH3)3CO-K+
- stability of alkoxides depends on solvation
bulky alkoxides are less solvated and less stable (stronger bases)
Oxygen Functional Groups
- alcohols are just the first of the many possible oxygen functional
groups
- oxidation leads to increasing number of bonds to oxygen
alkane --> alcohol --> carbonyl --> carboxyl -->
CO2
- reduction leads to decreasing number of bonds to oxygen
CO2 --> carboxyl --> carbonyl -->
alcohol --> alkane
Reactions of Alcohols
- acid/base reactions
- oxidation reactions
- substitution (C-O bond cleavage)
- elimination (dehydration)
Substitution Reactions with HX
- substitution by halogens using HX
OH is a poor leaving group
but initial protonation creates a good leaving group (H2O)
- halide substitution (SN1 mechanism)
tBuOH + HBr --> tBuOH2+ --> tBu+
--> tBuBr
favored by relatively stable carbocation (3°)
- halide substitution (SN2 mechanism)
MeOH + HBr --> MeOH2+ Br- --> MeBr
+ H2O
concerted displacement of H2O by Br- (unstable
carbocation)
- rearrangements are possible, even with 1° alcohols

Halogen Substitution with Other Reagents
- PBr3 + alcohol gives alkyl bromide,
with less rearrangement than with HBr
- SOCl2 + alcohol gives alkyl halide,
also with less rearrangement
Alkyl Sulfonates
- alcohol + RSO2Cl gives a sulfonate
ester
- sulfonate esters are excellent leaving groups
Dehydration (Elimination) Reactions
- acid-catalyzed dehydration: 3° > 2° >1°
favored by relatively stable carbocation,
absence of nucleophile, high temperature
- same first step as for substitution:
initial protonation creates a good leaving group (H2O)
- E1: carbocation loses H+ to form an alkene
tBuOH + H2SO4 -->
tBuOH2+ --> tBu+ --> (CH3)2C=CH2 + H+
- E2: less-substituted alcohols (unstable carbocations) show
concerted loss of H2O and H+
- rearrangements are commonly observed during dehydration
The Zaitsev Rule
- predicts regiochemistry of alkene formation
- the major product in an elimination reaction is the more
substituted alkene
(generally more stable)
- dehydration of an alcohol forms the more stable (more substituted)
alkene
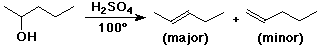
Pinacol Rearrangement
- acid-catalyzed dehydration of a glycol (1,2-diol),
with rearrangement
Alcohol Oxidation Reactions
- chromium (VI) oxidants:
CrO3, H2CrO4, K2Cr2O7
- oxidation involves intermediate chromate ester
- 1° alcohol to carboxylic acid with any Cr(VI) reagent,
e.g., CrO3
- 1° alcohol to aldehyde with PCC (avoids further oxidation)
- 2° alcohol to ketone with any Cr(VI) reagent
Glycol Oxidation Reactions
- periodic acid, H5IO6
(or HIO4·2H2O)
- C-C bond is cleaved and alcohols are oxidized
- intermediate is a cyclic periodic ester
- common degradation reaction for sugars
Thiol Reactions
- The most common preparation of thiols, RSH, depends on the
very high nucleophilicity of hydrosulfide ion, HS-
- Thiols are stronger acids than alcohols
- When dissolved an aqueous NaOH, they are converted completely
to alkylsulfide salts
- Thiols are oxidized to disulfides by a variety of oxidizing
agents, including O2. They are so susceptible
to this oxidation that they must be protected from air during
storage
- The most common reaction of thiols in biological systems
in interconversion between thiols and disulfides, -S-S-


![]()