
Chapter 2 Notes: Alkanes and Cycloalkanes

Hydrocarbons
- contain only C and H
- form basic carbon skeleton
- functional groups:
only C-H and C-C single bonds (alkanes)
C=C double bond (alkenes)
C=C triple bonds (alkynes)
rings (cycloalkanes, bicycloalkanes, polycycloalkanes)
aromatic rings (arenes)
Alkane Family
- methane CH4
- ethane CH3CH3
- propane CH3CH2CH3
- butane CH3CH2CH2CH3
- alkane CH3(CH2)nCH3
- CnH2n+2 (homologous series)
Higher Alkanes
- pentane C5H12
- hexane C6H14
- heptane C7H16
- octane C8H18
- nonane C9H20
- decane C10H22
- note the Greek prefixes
Alkane Isomers
straight-chain
branched-chain
cyclic chain
atoms connected in a different order
butane and isobutane
3 pentane isomers
Butane isomers
n-butane 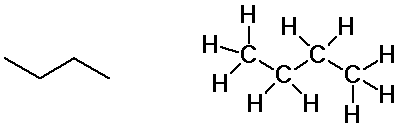
isobutane 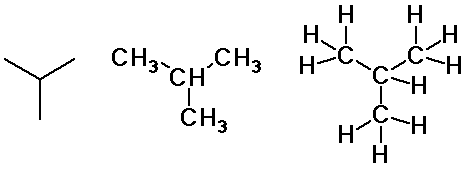
Pentane Isomers
- n-pentane
- isopentane
- neopentane
Alkyl Groups
n-propyl alcohol 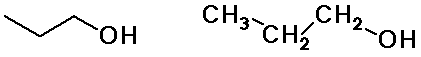
isopropyl alcohol 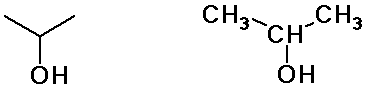
(constitutional isomers)
Butyl Groups
- n-butyl
- sec-butyl
- isobutyl
- tert-butyl
Classification of C Atoms
- 1° - primary - bonded to one other C
- 2° - secondary - bonded to 2 other C's
- 3° - tertiary - bonded to 3 other C's
- 4° - quaternary - bonded to 4 other C's
- class also applies to H atoms or functional groups attached
to that C
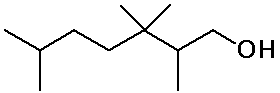
Identifying Carbon Classes
- identify the classes of each C, H and functional group
in the molecule below
IUPAC Nomenclature
(substituents)-(parent alkane)-(family)
Cl-CH2CH2-OH
2-chloro ethan ol
IUPAC Rules
- parent = longest continuous carbon chain
- number chain from end closest to a substituent (1st difference)
- assign numbers to each substituent
- in naming, arrange substituents alphabetically
- for multiple substituents, use prefixes di-, tri-, tetra-,
etc.
but they don't count when alphabetizing
- separate names from numbers with hyphens, numbers from
numbers with commas
- otherwise all written as one word
IUPAC Examples
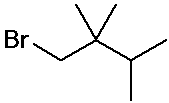
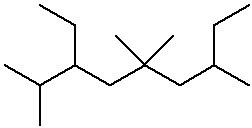
- name the three isomers of pentane
- name the four isomers of C4H9Br
IUPAC Examples
Cycloalkane nomenclature
Bicycloalkane nomenclature
- based on "bridgehead" positions
- [m.n.p] bicycloalkane
- m, n, p are the numbers of carbons in each bridge, listed
in decreasing order
- carbon numbers for substituents start at a bridgehead
and traverse larger bridges in order
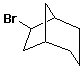 6-bromobicyclo[3.2.1]octane
6-bromobicyclo[3.2.1]octane
Writing Skeletal Structures
- omit C-H bonds
- assume C makes 4 bonds
- omit C atoms
- assume C at end of every bond
- especially useful for cyclic structures
- be able to put back all the details
Practice with Line Structures
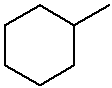 methylcyclohexane
methylcyclohexane
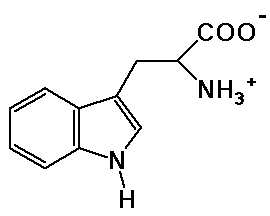 tryptophane
tryptophane
molecular formula ?
Conformations
- different 3D structures of the same molecule, differing
only by rotations about single bonds
Ethane Conformations
C-H bonds on the two carbons may or may not align
eclipsed: C-H bonds are aligned 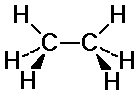
staggered: C-H bonds fit in between 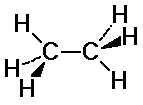
Newman Projections
- view down a C-C bond to visualize orientations
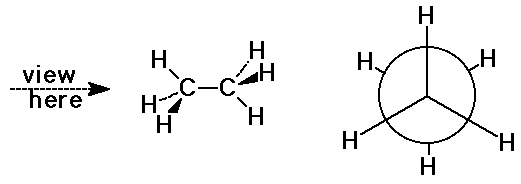
- staggered conformation (more stable)
Potential Energy Diagrams
- view energy changes as the C-C bond rotates
Butane conformations
- visualize rotation about the C2 - C3 bond
- monitor the relative positions of the two terminal methyl
groups
- gauche - dihedral angle ~60°
- anti - dihedral angle ~180°
- identify highest energy eclipsed conformation (dihedral
angle 0°)
Types of strain:
- torsional strain (staggered vs. eclipsed)
- nonbonded strain (gauche vs. anti)
- angle strain (small rings)
Small cycloalkanes
- cyclopropane - planar
- cyclobutane - slightly puckered
- cyclopentane - envelope or half-chair conformations
Cyclohexane
- chair conformations
- boat and twist-boat conformations
- potential energy diagram
Monosubstituted cyclohexanes
- axial and equatorial substituent locations
PRACTICE DRAWING ACCURATE CHAIR FORMS WITH SUBSTITUENTS
- relative stabilities of substituted cyclohexanes
equatorial generally preferred over axial
see the table in the text with delta G for axial vs equatorial
Disubstituted cyclohexanes - cis & trans isomerism
- numbered as 1,n
- cis - both substituents on the same side of the ring
- trans - substituents on opposite sides of the ring
- practice locating substituents in all the various combinations:
- cis-1,2-
- trans-1,2-
- cis-1,3-
- trans-1,3-
- cis-1,4-
- trans-1,4-
Depending on the substitution pattern, disubstituted cyclohexanes
have different combinations of axial and equatorial substituents.
|
substitution |
cis |
trans |
|
1,2- |
e,a <==>
a,e |
e,e <==>
a,a |
|
1,3- |
e,e <==>
a,a |
e,a <==>
a,e |
|
1,4- |
e,a <==>
a,e |
e,e <==>
a,a |
Example - the two chair conformations for cis-1-chloro-3-methylcyclohexane
involve either two axial substituents or two equatorial substituents.
The latter is more stable.
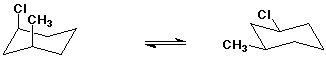
Alkanes - physical properties and sources
Reactions of alkanes
- combustion
- halogenation (later)

![]()
![]()
6-bromobicyclo[3.2.1]octane
![]() methylcyclohexane
methylcyclohexane tryptophane
tryptophane![]()
