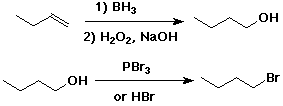1. (1 point each) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
a) ![]()
b) 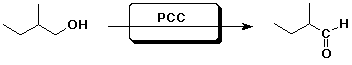
c) ![]()
2. (1 point each) Give a complete name for the following:
a) ![]()
(R)-1-bromo-5-hexen-2-ol
b) ![]()
(R)-4,4-dimethyl-2-cyclopentenethiol
3. (2 points) Write out the electron-pushing arrows that show the E2 dehydration of ethanol in concentrated sulfuric acid. Just show the rate-limiting step.
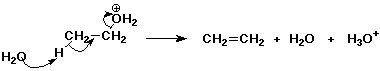
4. (3 points) Write out a sequence of reactions that would convert 1-butene to 1-bromobutane in good yield.