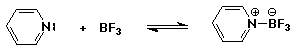1. (2 points each) Complete each of the following acid-base reactions. Use the table of pKa values to predict the preferred direction of the equilibrium (indicate to the RIGHT or to the LEFT).
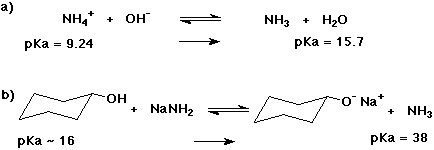
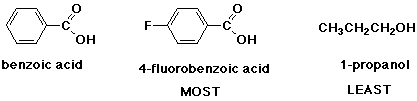
2. (2 points) Arrange the following in order of acidity. Indicate MOST under the most acidic and LEAST under the least acidic.
3. (2 points) Illustrate why the C-H bond adjacent to a ketone is relatively acidic by showing the resonance forms that stabilize the conjugate base of acetone.
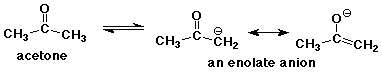
4. (2 points) Write a Lewis acid-base reaction between the following pair of compounds.