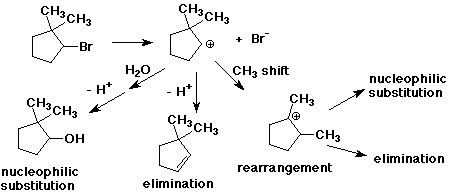
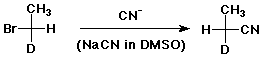1. (3 points) A carbocation can do three fundamentally different things: it can rearrange to another carbocation, it can react with a nucleophile to form a new bond, or it can eliminate a beta-hydrogen to form an alkene. Illustrate all three of these possibilities for the carbocation that would be formed by ionization of 2-bromo-1,1-dimethylcyclopentane.
2. (2 points) Synthetic substitution reactions are usually carried out under SN2 reaction conditions, since the reaction is stereospecific and avoids complications of carbocation intermediates (see above). Illustrate simple substitution reactions, starting from alkyl bromides, that could be used to make the following compounds:
a) 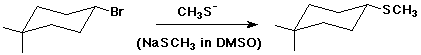
b)