Brown & Foote, pages 151 - 156 :
Problems 4.12, 15 - 29, 32 - 35
1. Write complete names for each of the following compounds, including designation of stereochemistry:
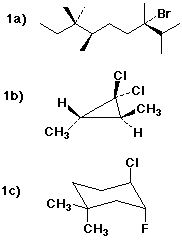
1a) (3S,6R)-3-bromo-2,3,6,7,7-pentamethylnonane
1b) (2S,3S)-1,1-dichloro-2,3-dimethylcyclopropane
1c) (3R,4R)-4-chloro-3-fluoro-1,1-dimethylcyclohexane
2. All of the naturally occuring amino acids are called L because they have a stereochemistry that was historically correlated with one stereoisomer of glyceraldehyde, shown below.
L-Glyceraldehyde and the natural amino acids all have the S absolute configuration. The two exceptions are glycine and cysteine. Look up their 3-dimensional structures and explain why they are different.
Glycine does not have a stereocenter (R = H).
Cysteine has a sulfur substituent (R = CH2SH), but it does have
the same relative configuration as all the other L-amino acids.
Because of the S atom, the priority for the R group is higher
than for the COOH group, which is different than for all the other
amino acids (where the R group priority falls between the COOH
and H).