1. (12 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) 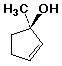
b) 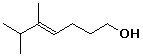
c) ![]()
2. (12 points) Write accurate structures that illustrate each of the following:
a) an SN2 transition state for bromide substitution on allyl iodide
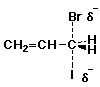
b) the most stable carbocation of composition C5H9
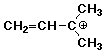
c) the conjugate acid of dimethyl disulfide
![]()
d) an isobutyl Grignard reagent
![]()
3. (16 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Indicate stereochemistry if it is specific.
If the reaction fits the description of SN1, SN2, E1, or E2, label it as such.
a) 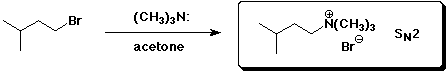
b) 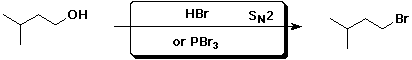
c) 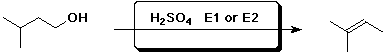
d) 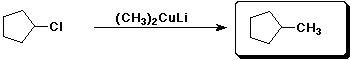
4. (10 points ) Write a complete mechanism for the solvolysis
of
1-bromo-1-methylcyclopentane in methanol as solvent.
Show all steps and all intermediates involved.
Use electron-pushing arrows to indicate electron flow for each
step.
Show both substitution and elimination products.

5. (10 points) Write a complete mechanism for the pinacol rearrangement shown below. Show all steps and electron-pushing arrows.

6. (15 points) Arrange the following in order with respect to the property indicated. Write "MOST" under the most reactive or highest value and "LEAST" under the least reactive or lowest value.
a) nucleophilicity
methanethiol / / t-butyl mercaptan / / t-butyl alcohol
MOST / / MID / / LEAST
b) reactivity in an SN2 mechanism
1-bromobutane / / 2-bromobutane / / 2-chlorobutane
MOST / / MID / / LEAST
c) reactivity in an SN1 mechanism
chlorocyclopropane / / chlorocyclobutane / / chlorocyclopentane
LEAST / / MID / / MOST (angle strain effects)
d) rate of dehydration using sulfuric acid
1-methylcyclohexanol / / cis-2-methylcyclohexanol / / cyclohexylmethanol
MOST / / MID / / LEAST
e) boiling point
perfluorocyclohexane / / perchlorocyclohexane / / cyclohexane
LEAST / / MOST / / MID
7. (15 points) Consider the two alternative mechanisms shown
below.
(2 pts) Write the overall reaction, which should be the same for
each mechanism.
(8 pts) Calculate H for each step in each mechanism and decide
which is the more likely mechanism.
(3 pts) For your more likely mechanism, identify the rate-determining
step, and write a transition state for that step.
(2 pts) Applying Hammond's Postulate, is this transition state
late or early?

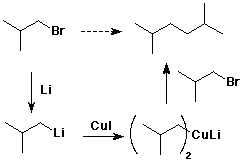
8. (10 points) Write out a sequence of reactions that could be used to accomplish one of the following conversions. Show the necessary reagents and conditions for each reaction step, but do not show mechanisms. Just do one of the two.