
Chapter 7 - Alkyl Halides

Structure
- alkyl halide: a compound containing a halogen atom
covalently bonded to an sp3 hybridized carbon atom
- given the symbol R-X
Vinylic and Aryl Halides
- if the halogen is bonded to an sp2 hybridized carbon, it
is a called a vinylic halide
- if it is bonded to a benzene ring, it is called an aryl halide,
given the symbol Ar-X
- we will not study vinylic and aryl halides in this chapter
Nomenclature
- halogen substituents are indicated by the prefixes fluoro-,
chloro-, bromo-, and iodo- and listed in alphabetical order with
other substituents
- for haloalkenes, numbering is determined by the location
of the C=C double bond
- common names - name the alkyl group followed by the name
of the halide
e.g., methyl chloride or chloromethane, allyl bromide or 3-bromopropene
- several polyhaloalkanes are common solvents and are generally
referred to by their common names
e.g., chloroform or trichloromethane, Freon 12 or dichlorodifluoromethane
- hydrocarbons in which all hydrogens are replaced by halogens
are commonly named as perhaloalkanes or perhaloalkenes
e.g., perfluoroethylene or 1,1,2,2-tetrafluoroethene
Dipole Moments
- dipole moment of RX depends on:
the sizes of the partial charges,
the distance between them, and
the polarizability of the unshared electrons on halogen
- dipole moments of all the haloalkanes are comparable
- see the table in your text
Van der Waals Forces
- van der Waals forces - a group of intermolecular forces,
including
dipole-dipole
dipole-induced dipole
induced dipole - induced dipole (dispersion forces)
- as atoms or molecules are brought closer together,
van der Waals attractive forces are overcome by
repulsive forces between electron clouds of adjacent atoms
- van der Waals radii
energy minimum is where net attractive forces are the strongest
- nonbonded interatomic and intermolecular distances at these
minima can be measured by x-ray crystallography
- each atom or group of atoms can be assigned an atomic or
molecular radius called a van der Waals radius
van der Waals radii
- see the table in your text
- notice that
F is only slightly larger than H
among the halogens, only I is larger than CH3
Boiling Points
- among constitutional isomers, branched isomers
have a more compact shape,
decreased area of contact,
decreased van der Waals attractive forces between neighbors,
and lower boiling points
- 1-bromobutane (n-butyl bromide) bp = 100°
- 2-bromo-2-methylpropane (t-butyl bromide) bp = 72°
- for an alkane and an alkyl halide of comparable size and
shape,
the alkyl halide has the higher boiling point
the difference is due almost entirely to the greater polarizability
of the three unshared pairs of electrons on halogen
compared with the polarizability of shared electron pairs of
the hydrocarbon
- ethane bp = -89°
- bromomethane bp = 4°
- boiling points of alkyl fluorides are lower than those of
hydrocarbons of comparable molecular weight
the difference is due to the small size of fluorine,
the tightness with which its electrons are held,
and their particularly low polarizability
- 2-methylpropane bp = - 1°
- 2-fluoropropane bp = - 11°
Density
- the densities of liquid alkyl halides are greater than those
of hydrocarbons of comparable molecular weight
a halogen has a greater mass per volume than a methyl or methylene
group
- some liquid alkyl chlorides are less dense than water
- all liquid alkyl bromides and iodides are more dense than
water
Bond Lengths, Strengths
- C-F bonds are stronger than C-H bonds
- C-Cl, C-Br, and C-I bonds are weaker
Halogenation of Alkanes
- if a mixture of methane and chlorine is kept in the dark
at room temperature, no change occurs
- if the mixture is heated, or exposed to visible or ultraviolet
light,
reaction begins at once with the evolution of heat

- what occurs is a substitution reaction,
in this case, substitution of a chlorine atom for a hydrogen
atom in methane
- substitution: a reaction in which an atom
or group of atoms is replaced by another atom or group of atoms
Regioselectivity
- regioselectivity of 2° hydrogen over a 1° hydrogen
is high for bromination
- propane + Br2 --> 1-bromopropane (8%) + 2-bromopropane
(92%)
- regioselectivity is not as high for chlorination
- propane + Cl2 --> 1-chloropropane (8%) + 2-chloropropane
(92%)
- regioselectivity is 3° > 2° > 1°
for bromination - approximately 1600:80:1
for chlorination - approximately 5:4:1
- example: draw all monobromination products for isobutane
and predict the % of each for a given reaction
Energetics
- using BDE data (Appendix 3), calculate the heat of reaction,
delta H°, for the halogenation of an alkane
- delta H = sum of bonds broken - sum of bonds made
Mechanism - A radical chain mechanism
- radical: any chemical species that contains one or more unpaired
electrons
- radicals are formed by homolytic cleavage of a bond
- a barbed curved (fishhook) arrow is used to show the change
in position of a single electron
Mechanism - Formation of radicals
- the order of stability of alkyl radicals is 3° > 2°
> 1° > methyl
- radical initiators like peroxides (ROOR) have weak bonds,
eaily broken
Mechanism - Chain initiation
- a step in a radical chain reaction characterized by formation
of radicals from nonradical compounds
Mechanism - Chain propagation
- a step in a radical chain reaction characterized by reaction
of a radical and a molecule to form a new radical
- Cl. + CH3CH3 --> CH3CH2. + HCl
- CH3CH2. + Cl2 --> CH3CH2Cl + Cl.
- notice that the radicals recycle
- chain length, n: the number of times the cycle of chain propagation
steps repeats in a chain reaction
Mechanism - Chain termination
- a step in a radical chain reaction that involves destruction
of radicals
- 2 Cl. --> Cl2
- CH3CH2. + Cl. --> CH3CH2Cl
- 2 CH3CH2. --> CH3CH2CH2CH3
Chain Propagation Steps
- for any set of chain propagation steps, their
equations add to the observed stoichiometry
energies add to the observed delta H°
Regioselectivity
- the regioselectivity of chlorination and bromination
can be accounted for in terms of the
relative stabilities of alkyl radicals (3° > 2° >
1° > methyl)
- how do we account for the greater regioselectivity of
bromination (1600:80:1) compared with chlorination (5:4:1)?
Hammond's Postulate
- What does a transition state look like?
- a transition state looks like something between reactants
and products
but closer in structure to whichever it is closer in energy to
- Hammond's Postulate: the structure of the transition state
for an exothermic reaction looks more like the reactants of that
step
for an endothermic reaction looks more like the products of that
step
- this postulate applies equally well to the transition state
for a one-step reaction
and to each transition state in a multi-step reaction
- in halogenation of an alkane, the rate-limiting step is hydrogen
abstraction
this step is exothermic for chlorination and endothermic for
bromination
- because hydrogen abstraction for chlorination is exothermic,
the transition state resembles the alkane and a chlorine atom,
there is little radical character on carbon in the transition
state, and
regioselectivity is only slightly influenced by radical stability
- because hydrogen abstraction for bromination is endothermic,
the transition state resembles an alkyl radical and HBr,
there is significant radical character on carbon in the transition
state, and
regioselectivity is greatly influenced by radical stability.
Radical stability
- 3° > 2° > 1° > methyl
- 3° C-H bond 93 kca/mol
- 2° C-H bond 96 kca/mol
- 1° C-H bond 100 kca/mol
- regioselectivity is in the same order
- the order is like carbocation stability
and for a similar reason -
the radical center is electron-deficient
and needs electron donation
Stereochemistry
- when radical halogenation produces a stereocenter
or takes place at a hydrogen on an existing stereocenter,
the product is an R,S mixture
- butane + Br2 --> racemic 2-bromobutane
- for simple alkyl radicals, the carbon bearing the radical
is sp2 hybridized
and the unpaired electron occupies the unhybridized 2p orbital
- since the radical is achiral, the Br attaches equally to
either side
Allylic Halogenation
- allylic carbon: a carbon adjacent to a C=C double bond
- allylic hydrogen: a hydrogen on an allylic carbon
- an allylic C-H bond is weaker than a vinylic C-H bond
- typical alkyl C-H 93-100 kcal/mol
- allylic C-H 86 kcal/mol
- vinylic or aryl C-H 106 kcal/mol
Allylic Bromination
- allylic bromination using NBS
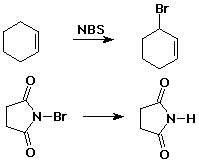
- the Br2 necessary for radical halogenation
is provided by reaction of NBS with HBr
- a radical chain mechanism
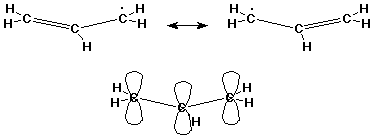
The Allyl Radical
- a hybrid of two equivalent contributing structures
Organometallic Compounds
- a compound that contains a metal bonded to a carbon atom
- Grignard reagents are formed by reaction of an alkyl halide
with magnesium metal in diethyl ether or tetrahydrofuran (THF)
- organolithium reagents are formed by reaction of an alkyl
halide
with lithium metal in a hydrocarbon solvent such as pentane
- carbon-metal bonds are polar covalent with negative carbon
- polarity (ionic vs. covalent character) depends on the metal
electronegativity
- organometallics are strong bases and react with any proton
donor stronger than
the alkane from which they are derived
- classes of proton donors that react with Grignard and organolithium
reagents are
alcohols, carboxylic acids, amines, thiols, alkynes, phenols,
water
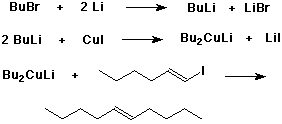
Gilman Reagents
- prepared from an organolithium reagent and copper(I) iodide
- Gilman reagents can be used to form new carbon-carbon bonds
by cross-coupling with alkyl or vinylic halides
![]()


