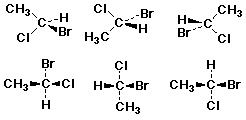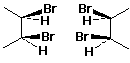Chapter 4 Notes: Stereochemistry

Stereochemistry
- chemistry in three dimensions
- includes both structure and reactivity effects
Enantiomers
- mirror-image stereoisomers
- like left and right hands
(see page 172 in your text)
- observed when a carbon atom has four different groups attached
to it
CHXYZ or CX1X2X3X4
Enantiomer Examples
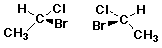
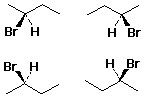
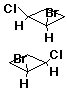
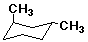
Chirality
- property of having "handedness"
(different from its mirror image)
- a molecule with any element of symmetry (e.g., a mirror plane)
must be achiral
Stereogenic Centers
- chiral centers or stereocenters
- a molecule with a stereogenic center (e.g., CX1X2X3X4) will
be chiral
- a stereogenic center cannot be:
sp- or sp2-hybridized (must be sp3)
an atom with 2 identical substituents (e.g., any -CH2- group)
Identifying Chiral Molecules
- achiral
- chiral

Properties of Enantiomers
- enantiomers have identical physical and chemical properties,
EXCEPT they
- interact with another chiral molecule differently
(like trying on left- or right-handed gloves - left and right
hands react differently)
- rotate the plane of plane-polarized light by equal amounts
but in opposite directions
Optical Activity
- chiral compounds rotate the plane of plane-polarized light
- rotation measured in degrees
clockwise (dextrorotatory or +) or
counterclockwise (levorotatory or -)
- polarimeter - instrument for measuring optical activity
Specific Rotation
- standard amount of optical rotation by 1 g/mL of compound
in a standard 1 decimeter (10 cm) cell
- [a] = a / l C
- where [a] is specific rotation
a = observed rotation in degrees
l = path length in dm
C = concentration in g/mL
Absolute Configuration
- nomenclature method for designating the specific arrangement
of groups about a stereogenic center
- differentiates between enantiomers
- uses the same sequence rules for establishing priority of
groups as was used for E and Z
R and S Designations
- assign priorities 1-4 (or a-d) to the four different groups
on the stereogenic center
- align the lowest priority group (4 or d) behind the stereogenic
carbon
- if the direction of a-b-c is clockwise, it is R
- if a-b-c is counterclockwise, it is S
Right- and Left-Hand Views
- textbook analogy - steering wheel
- alternative analogy - your hands
assign priorities to your fingers in order of height
a = middle finger, b = pointer finger, c = thumb, d = wrist
R - this works for your right hand
S - this works for your left hand
Drawing 3-D Structures
- practice with models
- dotted-line & wedge
- Fischer projections
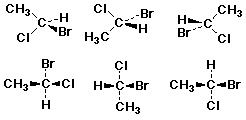
Fischer Projections
- a method for depicting stereochemistry at a series of chiral
centers
- arrange the chiral center so that:
- horizontal groups are forward
- vertical groups are oriented backward

- Note that there are numerous ways to show a given chiral
center
- 12 different Fischer projections represent (R)
- 12 different Fischer projections represent (S)
Multiple Stereogenic Centers
- compounds with more than 2 stereocenters have more than 2
stereoisomers
e.g., 2-bromo-3-chlorobutane
(2R,3R) and (2S,3S) are enantiomers
(2R,3S) and (2S,3R) are enantiomers
- in general, n stereocenters give 2^n stereoisomers
Diastereomers
- stereoisomers that are not enantiomers
e.g., (2R,3R) and (2R,3S)
(not mirror images, but not the same either)
- diastereomers may have different chemical and physical properties
Meso Compounds
- compounds with stereogenic centers but which are not chiral
e.g., (2R,3S)-2,3-dibromobutane
(same as its mirror image)
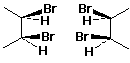
Identifying Meso Compounds
- mirror plane of symmetry
- one stereocenter is the mirror image of the other
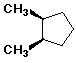
- cis-1,2-disubstituted cycloalkanes are meso if the two substituents
are identical
Cyclohexane Derivatives
- chair interconversions affect conformation, but not configuration
- trans-1,2-dichlorocyclohexane is (R,R) or (S,S)
- cis-1,2-dichlorocyclohexane is (R,S)
- one chair has the R stereocenter with axial Cl and S with
equatorial
- the other chair has R equatorial and S axial
- the two chair forms are enantiomers but not isolatable
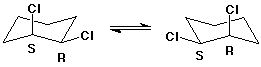
Racemic Mixtures
- an equal mix of both enantiomers (also called a racemate)
- a common form in the laboratory (but not in nature)
- optical resolution - separating enantiomers from a
mix (typically difficult)
Optical Purity / Enantiomeric Excess
- unequal mixtures of enantiomers may occur
- optical purity - compare actual rotation with what a pure
enantiomer would give (in %)
- enantiomeric excess - % excess of one pure enantiomer over
the other
- % optical purity = % enantiomeric excess
- example - consider a mix of 75% (R) + 25% (S)
- optical rotation would be 50% (50% inactive racemic + 50%
R)
- enantiomeric excess is also 50% (75% - 25%)
Optical Resolution
- for acids or bases - formation of diastereomeric salts from
a naturally ocurring acid or base
- enzymatic resolution - preferential binding or reaction of
just one enantiomer
Isomerism - Summary
- isomers - same molecular formula (same collection of atoms
used)
- constitutional isomers -differ in the connections between
atoms
different carbon skeletons
different functional groups
different locations of a functional group
Stereoisomers - Summary
- stereoisomers - same connections but in different 3D arrangement
- enantiomers - mirror-image stereoisomers
- diastereomers - non-mirror-image stereoisomers:
cis-trans diastereomers
other diastereomers
![]()


![]()