Organic Chemistry I |
Exam 3 Answer Key |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Exam 3 Answer Key |
Professor Carl C. Wamser |
![]()
1. (25 points) Write complete names for each of the following.
a) 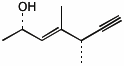
b) 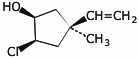
c) 
d) 
e) 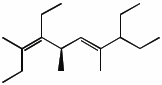
2. (15 points) Write accurate structures for the following:
a) the Lindlar hydrogenation product from pent-1-en-3-yne
![]()
b) (R,E)-3,5-dichlorooct-3-en-6-yne
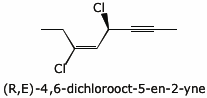
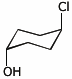
c) the chair conformation necessary for an E2 elimination from trans-4-chlorocyclohexanol
d) the SN2 transition state of (S)-2-iodobutane with hydroxide
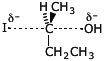
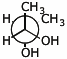
e) meso-butane-2,3-diol in a Newman projection
3. (15 points) Arrange the following in order with respect to the property indicated. Write “MOST” under the compound with the highest value and “LEAST” under the compound with the lowest value.
a) nucleophilicity
![]()
b) ratio of substitution over elimination with NaOH
![]()
c) SN2 reactivity

d) rate of E2 elimination with NaOH

e) number of chirality centers
![]()
4. (15 points) Complete each of the following reactions by adding the missing part: either the original reactant, the necessary reagents and conditions, or the final major product.
a) 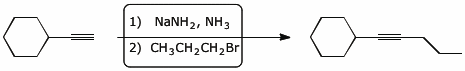
b) 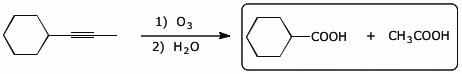
c) 
d) 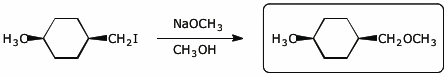
e) 
5. (10 points) Write a complete mechanism for the SN1 solvolysis reaction shown below. Show all steps and show electron-pushing arrows.
![]()

6. (10 points) Write the structure of (R,R)-2,3-dibromobutane in a Newman projection looking down the C2-C3 bond.
Treatment with KOH in EtOH leads to E2 elimination to form 2-bromo-2-butene. Predict whether the product will be (E) or (Z) or both.
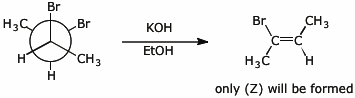
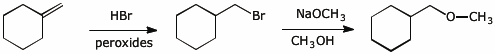
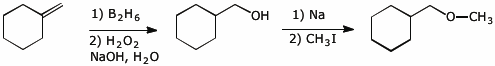
7. (10 points) Show a sequence of reactions that could be used to accomplish the following conversions.
a) prepare 2,2-dibromopropane , starting from acetylene and any alcohol you choose

b) 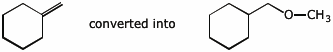
![]()