Organic Chemistry I |
Exam 1 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Exam 1 |
Professor Carl C. Wamser |
![]()
1. (15 points) Write complete names for each of the following.
a) (CH3)2CH(CH2)3CHBrCH2Br
b) 
c) 
2. (15 points) Write accurate structures for the following:
a) a Newman diagram that shows the anti conformation of pentane
b) the best conformation of trans-1,3-dimethylcyclohexane
c) two good resonance forms for HN3 (the three Ns are linear)
(show all lone pairs and formal charges)
d) 1,2,6-trimethylbicyclo[4.2.0]octane
e) 1,2,6-trimethylspiro[2.5]octane
3. (10 points) Refer to the compound shown below.
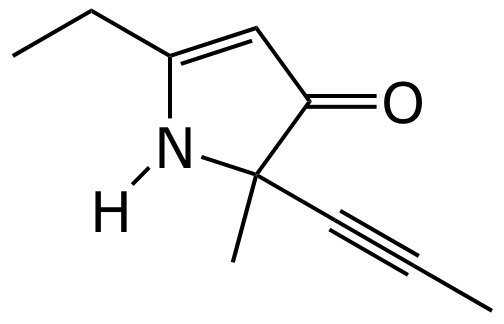
What is its molecular formula?
How many carbons are hybridized
sp _______________
sp2 _______________
sp3 _______________
For each sp3 carbon, identify its classification as 1°, 2°, 3°, or 4°.
Use arrows to specify each sp3 carbon on the structure above.
4. (14 points) Complete each of the following acid-base reactions. Identify the pKa values of the acids on each side of the equation and predict the preferred direction of the equilibrium.
a) ![]()
b) ![]()
c) ![]()
d) 
Of all the compounds cited above (on both sides of the equations), which is the strongest acid?
Which is the strongest base?
5. (18 points) Write line structures and complete names for all of the isomers of formula C5H10 that have one and only one ring.
6. (12 points) Complete the structures below by adding all the missing substituents at the appropriate places.
a) 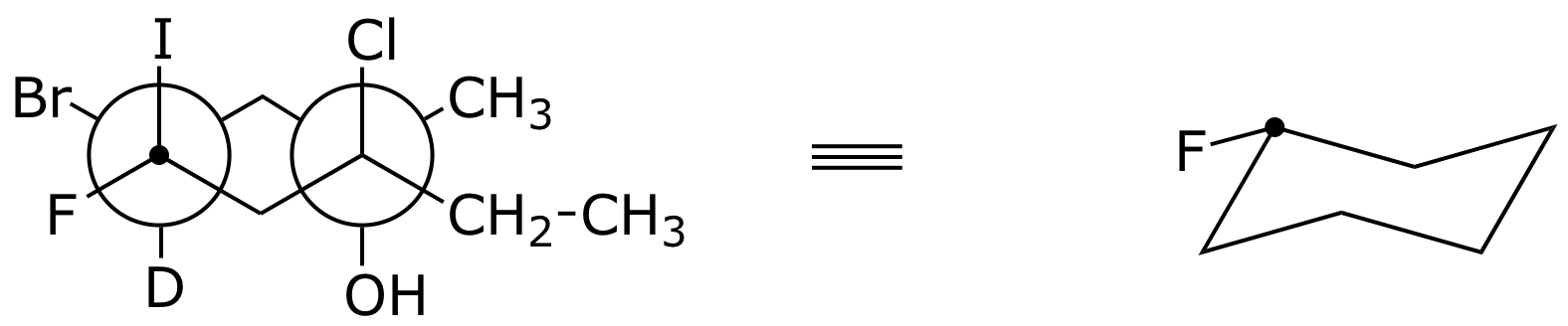
b) 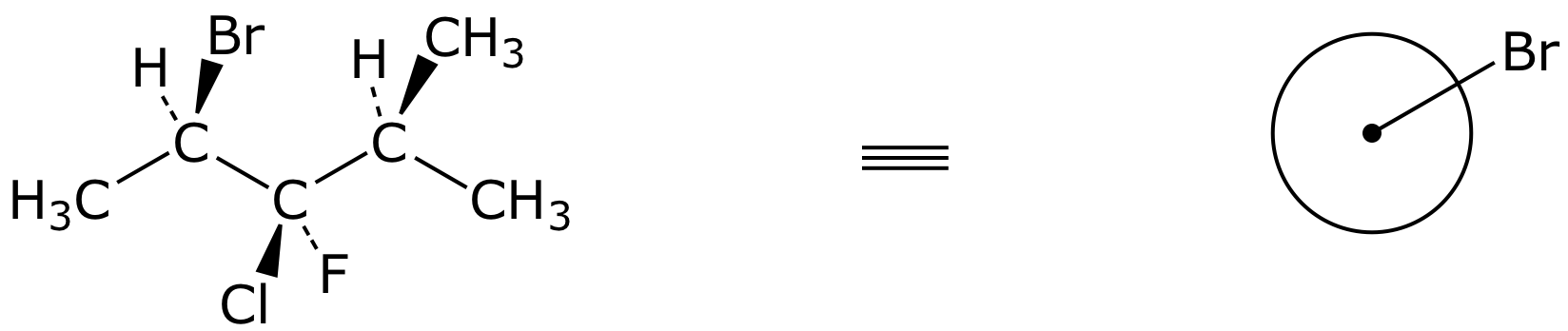
7. (16 points) Write Newman diagrams for all three staggered conformations of 3-ethyl-2-methylpentane, looking down the C2-C3 bond.
Identify the relative energies of the three forms.
Write one Newman diagram of the same compound, looking down the C3-C4 bond.
![]()