Organic Chemistry I |
Quiz 5 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Quiz 5 |
Professor Carl C. Wamser |
![]()
1. (2 points) Give a complete name for the following:
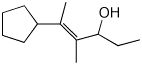
(E)-5-cyclopentyl-4-methylhex-4-en-3-ol
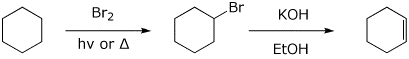
2. (2 points) Complete the following synthesis of cyclohexene from cyclohexane by showing the necessary reagents and conditions for each step.
3. (4 points) Write a complete mechanism, including electron-pushing arrows, for the reaction shown below.

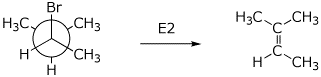
4. (2 points) Write a Newman diagram for 2-bromo-3-methylbutane, looking down the C2-C3 bond and showing Br anti to an H on C3.
Upon E2 elimination, what alkene product would be formed?
![]()