Organic Chemistry I |
Final Exam |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Final Exam |
Professor Carl C. Wamser |
![]()
1. (25 points) Write complete names for each of the following.
a) 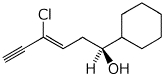
b) 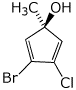
c) 
d) 
e) 
2. (15 points) Write accurate structures for the following:
a) ozonolysis products from 2-pentyne
b) the two propagation steps in the fluorination of ethane
c) 1,2,2-trimethylbicyclo[4.1.0]heptane
d) a repeat unit of PVC (polyvinylchloride)
e) the late transition state in SN2 hydrolysis of (R)-2-bromobutane in pure water
3. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) stability
![]()
b) boiling point
![]()
c) reactivity towards Br2
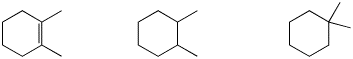
d) reactivity towards NaI
![]()
e) dehydration rate using H2SO4
![]()
4. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Include stereochemistry if it is specific.
a) 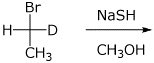
b) 
c) 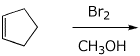
d) ![]()
e) ![]()
5. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Include stereochemistry if it is specific.
a) 
b) 
c) 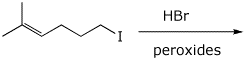
d) 
e) 
6. (20 points) Show sequences of reactions that you could use to convert the original alkanes into the products shown below.
a) 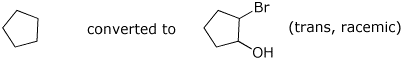
b) 
7. (20 points) Show a sequence of reactions that could be used to synthesize the compounds shown below. You may use acetylene and any alkanes having four or fewer carbons.
a) (hint - make 1-butyne)
![]()
b) (hint - make 3-hexyne)
![]()
8. (15 points) Calculate ?H values for the following reactions (using kcal/mol). Show the specific bond dissociation energies you use for your calculations.
a) ![]()
b) ![]()
c) ![]()
9. (20 points) Write Newman diagrams, viewing down the C1-C2 bond, for all three staggered conformations of (R)-1-bromo-1-chloropropane.
Upon E2 elimination with one equivalent of KOH in ethanol, only HBr is eliminated. Predict the relative energies of the three conformations, and predict the product expected from each conformation that can undergo E2 elimination of HBr, including which will be the major product.
10. (20 points) Starting with an optically pure sample of the compound below, write complete mechanisms, including electron-pushing arrows, for both an SN1 reaction and an E1 reaction.
Each mechanism should give two distinct products. Clearly show the expected stereochemistry in each case. Describe the relationship of the products to one another and the expected optical purity of the products in each case.
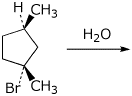
11. (20 points) In order to examine the regiochemistry and stereochemistry of a new reaction you have just discovered (addition of H-Z to C=C double bonds), you use the following compound as a test case.

Predict the product or products (addition only), including a description of the stereochemistry that would be expected in each of the following cases:
a) Markovnikov regiochemistry, nonspecific stereochemistry
b) Markovnikov regiochemistry, anti stereochemistry
c) anti-Markovnikov regiochemistry, syn stereochemistry
d) anti-Markovnikov regiochemistry, anti stereochemistry
![]()