Organic Chemistry I |
Exam 3 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Exam 3 |
Professor Carl C. Wamser |
![]()
1. (20 points) Write complete names for each of the following.
a) 
b) 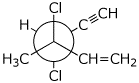
c) 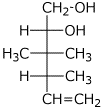
d) 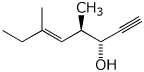
2. (15 points) Write accurate structures for the following:
a) meso-3,5-heptanediol
b) lithium acetylide
c) (R,R)-1,3-cyclopentanediol
d) DMSO or tosyl chloride (your choice)
e) (Z)-2-hexen-4-yne
3. (10 points) Complete each of the following structures on the right by adding what would be needed to make them the identical compound as shown on the left.
a) 
b) 
c) 
d) 
e) 
4. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) acidity
![]()
b) nucleophilicity
![]()
c) rate of hydrolysis
![]()
d) ratio of substitution/elimination using KOH/EtOH
![]()
e) amount of (S) product expected, starting with (R)-2-bromobutane
![]()
5. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. For the first three reactions, specify the expected stereochemistry of the product.
a) 
b) 
c) 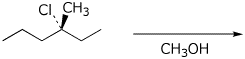
d) 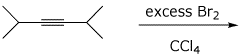
e) ![]()
6. (10 points) Show the sequence of reactions you would use to synthesize the epoxide shown below, starting with acetylene and bromomethane as your sources of carbon.
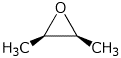
7. (15 points) For the formation of a bromohydrin from cis-2-butene, write a complete mechanism, carefully tracking the stereochemistry at each of the steps. Label stereocenters as R or S throughout your mechanism.

![]()