Organic Chemistry I |
Final Exam Answer Key |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Final Exam Answer Key |
Professor Carl C. Wamser |
![]()
1. (25 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 
b) 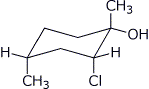
c) 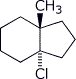
d) 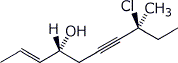
e) 
2. (18 points) Write accurate structures for the following:
a) the best chair conformation of cis-1,4-dimethylcyclohexane

b) the conjugate base of 4-methyl-1-pentyne

c) a Newman diagram of the best conformation of isobutyl alcohol, looking down the C1-C2 bond
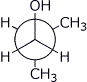
d) the repeat unit (show at least two) of poly(tetrafluoroethylene) (Teflon)

e) the weakest C-H bond in 2,2,3-trimethylpentane

f) (R,Z)-hex-3-en-5-yn-2-ol
![]()
3. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) stability
![]()
MIDDLE / / / MOST / / / LEAST
b) nucleophilicity
![]()
MIDDLE / / / MOST / / / LEAST
c) reactivity in an SN2 mechanism
![]()
MIDDLE / / / LEAST / / / MOST
d) boiling point
![]()
MIDDLE / / / MOST / / / LEAST
e) reactivity towards concentrated sulfuric acid
![]()
MIDDLE / / / MOST / / / LEAST
4. (12 points) Complete each of the following structures on the right so that they represent the identical compound to the structure on the left in each case.
a) 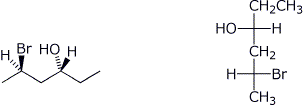
b) 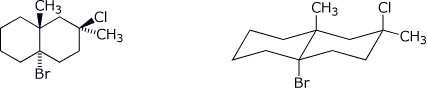
c) 
5. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 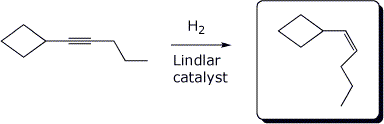
b) 
c) 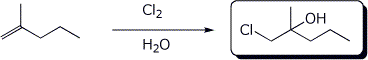
d) 
e) 
6. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 
b) 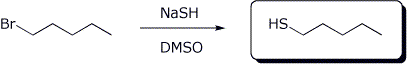
c) 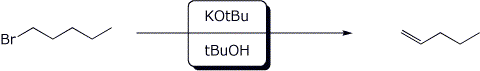
d) 
e) 
7. (15 points) Fill in the missing components in the synthetic sequence below.
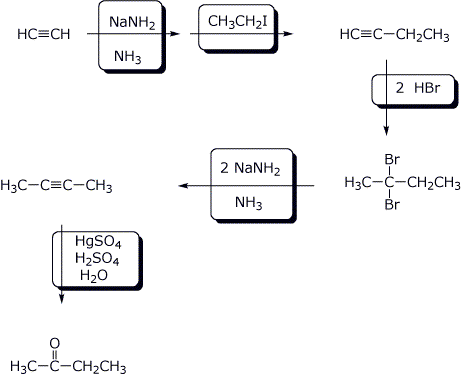
8. (15 points) Fill in the missing components in the synthetic sequence below.
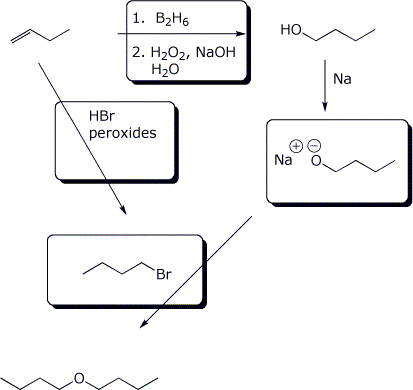
9. (15 points) Fill in the missing components in the synthetic sequence below.
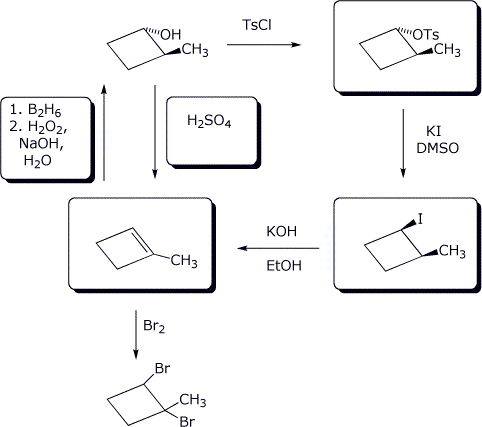
10. (20 points) Starting with cis-1,3-dimethylcyclopentane, photobromination leads to one primary product, 1-bromo-1,3-dimethylcyclopentane. Write out this reaction, initially ignoring stereochemistry issues.

Follow the absolute stereochemistry by writing clear structures of the original compound, the expected intermediate(s), and the final product(s). Show R&S designations for every structure. Predict whether the final product will be a mixture of enantiomers, diastereomers, or other relationships.

11. (20 points) Starting with (R)-2-iodobutane in water, possible reaction pathways include SN1, SN2, E1, and E2. Write detailed mechanisms for each of these. For the elimination mechanisms, just show the pathway to the major product. Be specific about stereochemistry in each case.
SN1

SN2
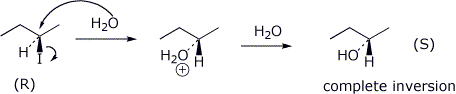
E1
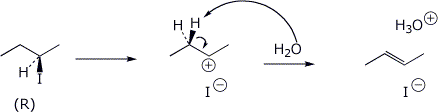
E2

12. (15 points) One of the steps in the citric acid (or Krebs) cycle involves the enzyme-catalyzed hydration of fumaric acid to give malic acid. The absolute stereochemistry of the reactant and product are as shown below.

In order to determine whether the addition is syn or anti, experiments were performed with D2O. Predict the specific stereochemistry of the product (note that there will be two chirality centers in the possible products) if the addition is syn and if the addition is anti. Write complete structures for all products expected and show R & S designations on all stereocenters.
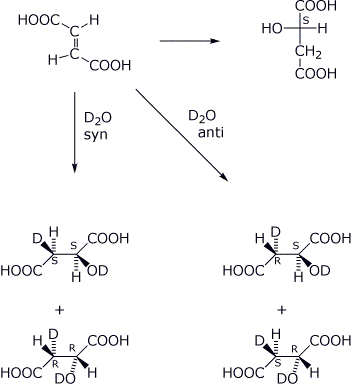
![]()