Organic Chemistry I |
Exam 2 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Exam 2 |
Professor Carl C. Wamser |
![]()
1. (20 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) ![]()
b) 
c) 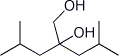
d) 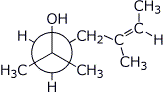
2. (15 points) Write accurate structures for the following:
a) the chlorohydrin from propene
b) protonated 1-methylcyclohexanol
c) the repeat unit of polypropylene
d) triethylborane
e) (E)-2-bromo-1,3-dichloro-1-butene
3. (15 points) Arrange
the following in order with respect to the property indicated.
Write MOST and
LEAST under the compounds with the highest and lowest values, respectively.
a) carbocation stability
1-methylcyclopentyl 3-methylcyclopentyl cyclopentylmethyl
b) rate of reaction with H2SO4
1-methylcyclopentanol 3-methylcyclopentanol cyclopentylmethanol
c) stability
1-methylcyclopentene 3-methylcyclopentene 1,2-dimethylcyclopentene
d) reactivity towards Br2
1-methylcyclopentene 3-methylcyclopentene 1,2-dimethylcyclopentene
e) reactivity towards Br2
1-methylcyclopentene methylcyclopentane cyclopentane
4. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 
b) ![]()
c) 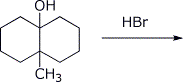
d) 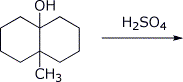
e) 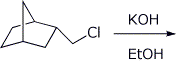
5. (15 points) Use the table of bond dissociation energies to calculate delta H for each of the two reactions below. Predict which reaction is likely to be more favorable based on that calculation. Use kcal/mol.
a) ![]()
b) ![]()
Write a general mechanism that would fit both reactions (use HX to represent
HF or HI).
Based on your mechanism, predict which reaction is likely to be
more favorable. Explain.
6. (10 points) The photochemical chlorination of 2,2,3-trimethylbutane gives
just three products.
Write their structures and predict their relative amounts,
assuming that the reactivity of chlorine atoms with C-H bonds is in the order
3° > 2° > 1° in the ratio 5 : 4 : 1 .
7. (10 points) Addition
of HX to an alkene typically does not show any special stereochemistry. For
example, addition of HCl to 1,2-dimethylcyclopentene gives two stereoisomeric
products that could be described as arising from syn addition or anti addition.
Show the overall reaction and the structures of the two products, identifying
each as the result of syn or anti addition.
In fact, one stereoisomer predominates in the product mixture. Write a complete mechanism and predict which product will be the major product.
![]()