Organic Chemistry I |
Final Exam |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Final Exam |
Professor Carl C. Wamser |
![]()
1. (25 points) Write complete names for each of the following, including stereochemistry
a) 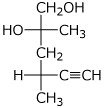
b) 
c) 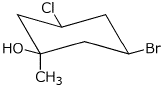
d) 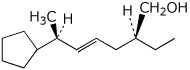
e) 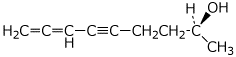
2. (15 points) Write accurate structures for the following:
a) isobutyl radical
b) the two propagation steps in the chlorination of ethane
c) 2-methylbicyclo[4.2.0]octane
d) the most stable conformation of 1,1,3-trimethylcyclohexane
e) the Zaitsev elimination product from 1-bromo-2-methyl cyclopentane
3. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) boiling point
![]()
b) reactivity with iodide
![]()
c) rate of reaction
![]()
d) nucleophilicity
![]()
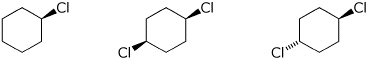
e) amount of axial Cl at equilibrium
4. (15 points) Complete each of the following reactions, predicting the major product. Show stereochemistry if it is specific.
a) 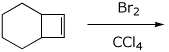
b) 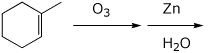
c) 
d) 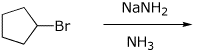
e) 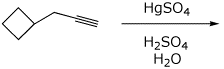
5. (15 points) Complete each of the following reactions, predicting the major product. Show stereochemistry if it is specific.
a) ![]()
b) 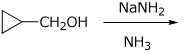
c) 
d) 
e) 
6. (15 points) Complete each of the following structures by adding the missing components on the right. In each case, these are meant to be identical structures, viewed from different perspectives.
a) 
b) 
c) 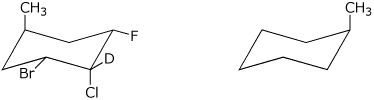
7. (15 points) Write Newman projections (looking down the C2-C3 bond) for the three staggered conformations of (R)-2-chloro-3-methylbutane.
Show which conformation could lead to a Zaitsev E2 elimination, and predict the product that would be formed.
What Zaitsev E2 elimination product would be formed if the original compound were (S)?
8. (15 points) Use the table of bond dissociation energies to predict ΔH for each of the following reactions.
a) ![]()
b) ![]()
c) ![]()
9. (20 points) Show the steps that would be needed for the following synthetic transformations. Show each separate reaction and product, but mechanisms are not needed.
a) 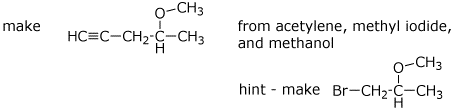
b) 
10. (15 points) Write a complete mechanism for the addition of Br2 to cis-2-butene, including the stereochemistry at each stage. Show each step with electron-pushing arrows and designate the absolute stereochemistry at all chirality centers.
11. (15 points) Free radical chlorination of 1,1-dimethylcyclobutane gives several monochlorination products. Write names and structures for each product.
Predict the relative amounts of each product, assuming the reactivity of Cl• with different C-H bonds follows the order 3° > 2° > 1° by a ratio of 5 : 4 : 1.
For products that are chiral, predict whether they would be formed optically active or not.
12. (20 points) Hydrolysis of 3-bromo-4-methylhexane gives both 4-methyl-3-hexanol
and
3-methyl-3-hexanol. Write out the reaction as described, initially ignoring
stereochemistry.
Starting with the original compound as the (R,R) stereoisomer, write out a
complete mechanism that shows the formation of both products and indicates
the expected stereochemistry of each of the two products.
![]()