Organic Chemistry I |
Exam 3 Answer Key |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Exam 3 Answer Key |
Professor Carl C. Wamser |
![]()
1. (15 points) Write complete names for each of the following, including stereochemistry
a) 
(R,Z)-4-bromohept-5-en-1-yne
b) 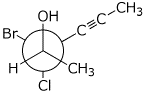
(2R,3S)-3-bromo-3-chlorohex-4-yn-2-ol
c) 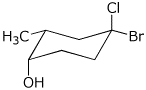
(1S,2R,4R)-4-bromo-4-chloro-2-methylcyclohexanol
2. (15 points) Write accurate structures for the following:
a) a polar aprotic solvent
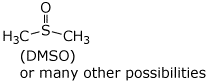
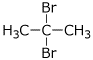
b) two enantiomers of C2H4BrCl

c) a meso compound of formula C5H8Br2 (no double bonds)

d) the conjugate base of 1-pentyne
![]()
e) the geminal dibromide from addition of HBr to 1-pentyne
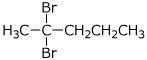
3. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) rate of reaction with KI in DMSO
![]()
LEAST / / / MIDDLE / / / MOST
b) rate of reaction with CH3OH

LEAST / / / MOST / / / MIDDLE
c) nucleophilicity
![]()
LEAST / / / MIDDLE / / / MOST
d) acidity
![]()
MIDDLE / / / LEAST/ / / MOST
e) ratio of substitution / elimination using KOH in EtOH

LEAST / / / MIDDLE / / / MOST
4. (15 points) Complete each of the following reactions, predicting the major product. Show stereochemistry if it is specific. Also indicate the expected mechanism (e.g., SN2).
a) 
b) 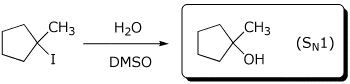
c) 
d) 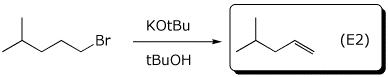
e) 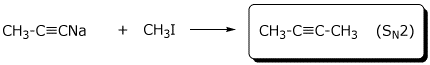
5. (15 points)
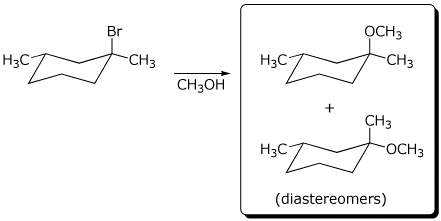
a) The reaction below gives two stereoisomers of formula C9H18O .
Draw their structures.
Are they enantiomers or diastereomers?
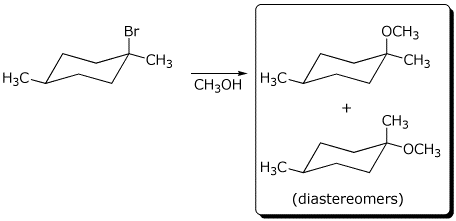
b) The reaction below gives two stereoisomers of formula C9H18O .
Draw their structures.
Are they enantiomers or diastereomers?
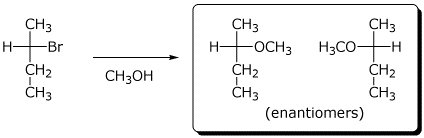
c) The reaction below gives two stereoisomers of formula C5H12O .
Draw their structures.
Are they enantiomers or diastereomers?
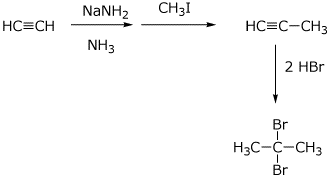
6. (10 points) Show the steps that would be used to synthesize the target compound below, starting with acetylene and methyl iodide as the only sources of carbon. You may use any needed reagents, solvents or inorganic materials.
7. (15 points) The following stereospecific synthesis was carried
out, starting with optically active 1-bromo-1-deuteroethane and ending with
optically active 2-deuterobutane.
( D = deuterium = the hydrogen isotope of mass 2 )

If the original compound had S stereochemistry, write out the two reactions above to show the expected stereochemistry of the reactants and products at each stage.
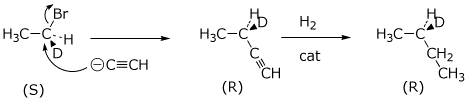
If optically pure (S)-2-deuterobutane is known to have a specific rotation of [a] = +10°, and the final product is observed to have [a] = -9°, what can be concluded about the product with respect to its
a) optical purity = 90%
b) enantiomeric excess = 90%
c) composition in % (R) and % (S) = 95% (R) + 5% (S)
If the original 1-bromo-1-deuteroethane was known to be 90% optically pure (S) enantiomer, what can you conclude about the stereochemistry of each of the two reactions?
a) reaction 1 with NaC=CH
complete inversion of stereochemistry
b) reaction 2 with H2
complete retention of stereochemistry
![]()