Organic Chemistry I |
Exam 2 |
Professor Carl C. Wamser |
![]()
Organic Chemistry I |
Exam 2 |
Professor Carl C. Wamser |
![]()
1. (15 points) Write complete names for each of the following.
a) 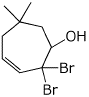
b) 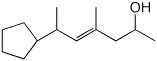
c) 
2. (15 points) Write accurate structures for the following:
a) the conjugate base of sec-butyl alcohol
b) the epoxide from cyclopentene
c) a late transition state in the reaction of CH4 with
Br•
d) the SN2 transition state in the reaction of CH3OH
with HI
e) a C5H12 isomer that would
give only one monochlorination product
3. (15 points) Arrange the following in order with respect to the property indicated. Write MOST and LEAST under the compounds with the highest and lowest values, respectively.
a) reactivity towards Br2
b) rate of dehydration
c) exothermicity (hint - BDE table)
d) stability
isobutyl radical / / / sec-butyl radical / / / tert-butyl radical
e) stability
4. (15 points) Complete each of the following reactions by adding
the missing part: either the starting compound, the necessary reagents and
conditions, or the final major product.
Show stereochemistry if it is specific.
a) 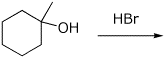
b) 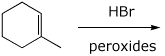
c) 
d) 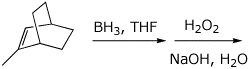
e) 
5. (15 points) Consider the two reactions shown below.
Do these two reactions constitute propagation steps for a chain mechanism?
If so, what is the overall balanced reaction?
Calculate ΔH for each of the two steps.
Write an approximate potential energy diagram for step 1 and step 2 (one diagram that shows the two reactions following one other). Show where your ΔH values would be measured.
6. (10 points) Write a complete mechanism for the reaction shown below. Show all steps and electron-pushing arrows for each step.
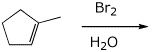
7. (15 points) Complete the following synthetic sequence by adding the missing components.
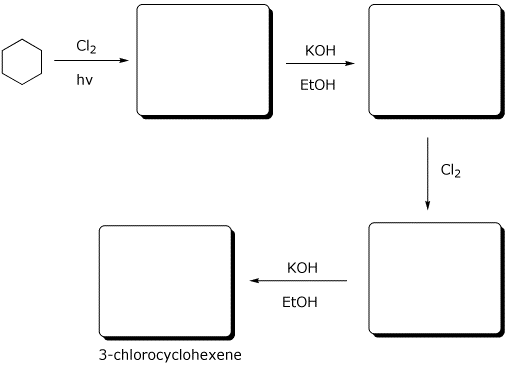
The final product ( 3-chlorocyclohexene ) is formed in good yield when just one equivalent of KOH is used. What product would be expected if 2 equivalents of KOH were used?
Show a clear chair structure that illustrates why the alternative product, 1-chlorocyclohexene, is not formed.
![]()