|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 2 Quiz |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 2 Quiz |
![]()
1. (3 points) What is the molecular formula of the compound shown below?
C9 H12 O
How many carbons are hybridized
sp ___0____ , sp2 ___5____ , sp3 ____4___
Put ( * ) next to every sp3 carbon that is primary ( 1° ).
Put ( • ) next to every sp3 carbon that is secondary ( 2° ).
2.
(3 points) Arrange the following in order with respect to the property
indicated.
Write MOST and LEAST under the compound with the highest and lowest values.
a) boiling point
b) water solubility
c) stability
3. (4 points) Give a complete name for each of the following compounds:
a) 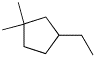
3-ethyl-1,1-dimethylcyclopentane
b) 
2-bromo-4-chloro-4-methylhexane
![]()