|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 1 Quiz |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 1 Quiz |
![]()
1. (4 points) HOCN and HNCO are isomers that are readily interconverted
in base.
Complete the four boxes below with accurate Lewis structures, showing
all bonds and all lone pairs.
2. (2 points) Write a good Lewis structure for the compound HOF, showing
all bonds and all lone pairs.
Using the VSEPR approach, predict the geometry
of the electron distribution about O and the molecular (nuclear) structure
about O.
Electron distribution is tetrahedral.
Molecular structure is bent.
3. (4 points) Ammonia dissolves in methanol and undergoes some ionization.
Write two acid-base reactions that could occur, and predict the preferred
direction of each equilibrium (RIGHT or LEFT).
Potentially relevant pKa data are:
NH3 (36) , NH4+ (9) , CH3OH (15) , CH3OH2+ (-2)
a) 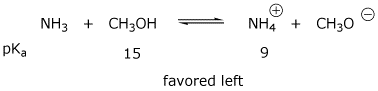
b) 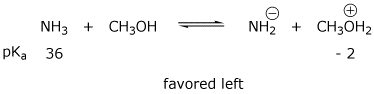
![]()