|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 2 |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 2 |
![]()
1. (15 points) Write complete names for each of the following.
a) 
b) 
c) 
2. (15 points) Write accurate structures for the following:
a) ethyloxonium ion
b) a (Z) alkene of formula C3H5Br
c) the major product from bromination of methylcyclopentane
d) the chlorohydrin from cyclohexene
e) a termination reaction step in the chlorination of methane
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 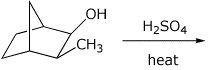
b) 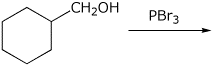
c) 
d) 
e) 
4. (15 points) Arrange the following in order with respect to the property
indicated.
Write MOST and LEAST under the compounds with the highest and
lowest values, respectively.
a) rate of dehydration

b) polarizability
![]()
c) rate of E2 elimination
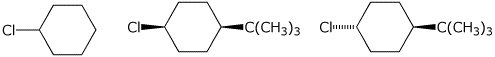
d) stability

e) earliest transition state
![]()
5. (8 points) Complete the following synthetic sequence by adding the missing parts: either the starting compound, the necessary reagents and conditions, or the final major product.

6. (10 points) Write a complete mechanism for the reaction shown below. Show all steps and electron-pushing arrows for each step.

7. (10 points) Only two products are observed upon the free radical chlorination
of
1,1-dimethycyclopropane.
Write names and structures for the two monochlorination
products expected.
Predict the relative amount of each of the products, given that the relative
reactivity of Cl• for
different C-H bonds is in the order of 3° > 2° > 1° by
a ratio of 5 : 4 : 1 .
8. (12 points) You have discovered a new reagent that apparently adds to C=C double bonds. To test its regiochemistry and stereochemistry, you react it with 1-methylcyclopentene and observe the major product shown below.
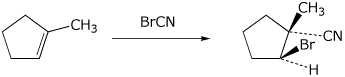
You would describe the stereochemistry of the addition as _______________ .
You want to correlate the regiochemistry with Markovnikov’s Rule, so you describe the electrophile and nucleophile as follows.
_____________________________ acts as an electrophile
_____________________________ acts as a nucleophile
Write a complete mechanism for your proposed reaction, in particular explaining why the observed regiochemistry and stereochemistry occur.
![]()