|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 1 |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 1 |
![]()
1. (15 points) Write complete names for each of the following.
a) 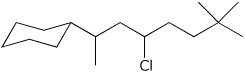
b) 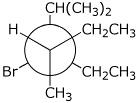
c) 
2. (15 points) Write accurate structures for the following:
a) the best Lewis structure for H2CN2 ( C-N-N is linear )
b) two good resonance forms for the SCN anion ( S-C-N is linear )
c) the most stable chair conformation of trans-1,2-dimethylcyclohexane
d) a bicyclo[2.2.1]heptane that has a quaternary carbon atom (substituents are allowed)
e) the isomer of C6H14 that has ONLY primary and tertiary carbons
3a. (12 points) Write structures and names for all the possible isomers of C3H6Cl2 .
3b. (12 points) Write structures and names for all the possible isomers
of
dichlorocyclopropane.
4. (15 points) Write Newman diagrams for all three staggered
conformations of
3-bromo-2-methylpentane. Carbon-1 is shown on the first diagram to get you
started.
5. (16 points) Complete the following acid-base reactions and predict whether the equilibrium will be favored to the right or to the left.
a) ![]()
b) ![]()
c) ![]()
d) ![]()
6. (15 points) Shown below is a chair conformation and a Newman diagram
of the same structure.
Carbons 1 and 3 are identified on each to help orient
the two views.
Place the following substituents at the indicated locations on each of the
two structures:
an equatorial methyl on C-1
an axial Br on C-3
an equatorial methyl on C-4
an axial F on C-6
Write the other chair conformation of this same compound (with all four substituents) in both a Newman and a chair structure.
![]()