|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 8 Quiz |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 8 Quiz |
![]()
1. (3 points) Write the structure of (S)-1-bromo-2-methylbutane and give the name and structure for the product that would be expected from its reaction with sodium iodide in acetone. What mechanism is involved?
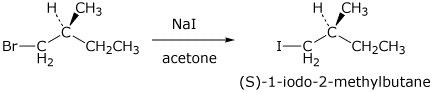
2. (4 points) Write a complete mechanism for the solvolysis reaction of
1-bromo-1-methylcyclopentane in methanol solvent. Both a substitution and
an elimination product are formed - show pathways to both products.

3. (3 points) Show a mechanism for the reaction shown below.
The cis isomer of the compound shown below does not do the same reaction.
Explain why not.
![]()