|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 2 Quiz |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 2 Quiz |
![]()
1. (3 points) On the compound below, put circles around all the primary (1°) carbons, and put an asterisk (*) next to all quaternary (4°) carbons.
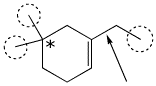
Name the hybrid orbitals used to make the bond shown by the arrow.
C(sp2) - C(sp3)
2. (4 points) Give complete IUPAC names for each of the following compounds:
a) 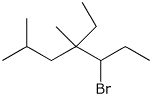
b) 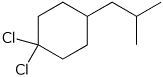
3. (2 points) Write a structure for 1,1-di(tert-butyl)cyclopropane.
Show all carbons and hydrogens, but 3D is not required.

4. (1 points) Among the isomers of pentane, the one with the lowest boiling point is (give a name or a structure).

![]()