|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Final Exam Answers |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Final Exam Answers |
![]()
1. (25 points) Write complete names for each of the following.
a) 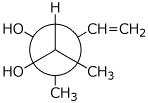
b) 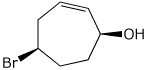
c) 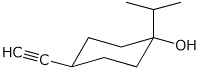
d) 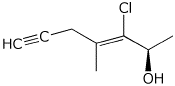
e) 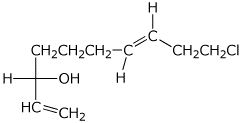
2. (15 points) Write accurate structures for the following:
a) gauche 1-iodopropane
b) the Zaitsev product of elimination from 3-bromo-2-methylheptane
c) the Markovnikov product from addition of HBr to 2-methyl-2-heptene
d) a compound with an sp carbon but NO triple bonds
e) the polymer from chloroethene (write at least two repeat units)
3. (15 points) Arrange each of the following in
order with respect to the property indicated.
Write “MOST” under the compound with the highest value and “LEAST” under
the compound with the lowest value.
a) acidity
LEAST / / MIDDLE / / MOST
b) basicity
MOST / / LEAST / / MIDDLE
c) C-H bond strength
MOST / / MIDDLE / / LEAST
d) SN1 reactivity
LEAST / / MIDDLE / / MOST
e) carbocation stability
MIDDLE / / LEAST / / MOST
4. (15 points) Complete the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the major product expected. Show stereochemistry if it is specific.
a) 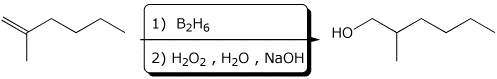
b) 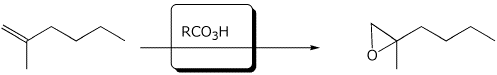
c) 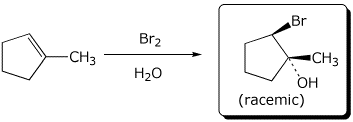
d) 
e) 
5. (20 points) Complete the following reactions by indicating the major product expected. Show stereochemistry if it is specific. Indicate whether the expected mechanism to the major product will be SN1, SN2, E1, or E2.
a) 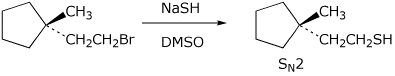
b) 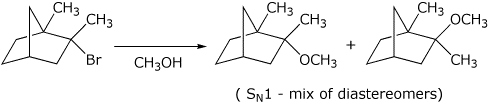
c) 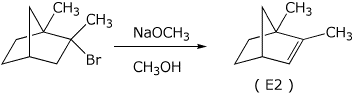
d) 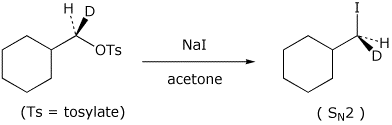
e) 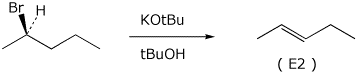
6. (15 points) Complete each of the following acid-base reactions and identify whether the equilibrium will be favored to the right or to the left.
a) ![]()
b) ![]()
c) ![]()
d) ![]()
e) ![]()
7. (20 points) Calculate ΔH for each of the following reactions. Clearly indicate the bonds being broken and the bonds being made for each reaction.
a) ![]()
broken: 2° C-H (95 kcal or 397 kJ) + Br-Br (46 kcal or 192 kJ)
made: 2° C-Br (68 kcal or 284 kJ) + H-Br (87.5 kcal or 366 kJ)
ΔH = 95 + 46 - 68 - 87.5 = -14.5 kcal/mol
ΔH = 397 + 192 - 284 - 366 = -61 kJ/mol
b) ![]()
broken: CH3-CH3 (88 kcal or 368 kJ) + H-Cl (103 kcal or 431 kJ)
made: CH3-H (104 kcal or 435 kJ) + CH3-Cl (83.5 kcal or 349 kJ)
ΔH = 88 + 103 - 104 - 83.5 = +3.5 kcal/mol
ΔH = 368 + 431 - 435 - 349 = +15 kJ/mol
c) ![]()
broken: CH3-OH (91 kcal or 380 kJ) + H-Cl (103 kcal or 431 kJ)
made: HO-H (119 kcal or 497 kJ) + CH3-Cl (83.5 kcal or 349 kJ)
ΔH = 91 + 103 - 119 - 83.5 = -8.5 kcal/mol
ΔH = 380 + 431 - 497 - 349 = -35 kJ/mol
d) ![]()
broken: CH3-OH (91 kcal or 380 kJ) + H-Br (87.5 kcal or 366 kJ)
made: HO-H (119 kcal or 497 kJ) + CH3-Br (70 kcal or 293 kJ)
ΔH = 91 + 87.5 - 119 - 70 = -10.5 kcal/mol
ΔH = 380 + 366 - 497 - 293 = -44 kJ/mol
e) The ΔH values obtained from reactions c & d above do NOT allow you to predict whether HCl or HBr reacts faster with methanol. Explain why not.
In fact, HBr reacts much faster than HCl does with methanol. Explain why.
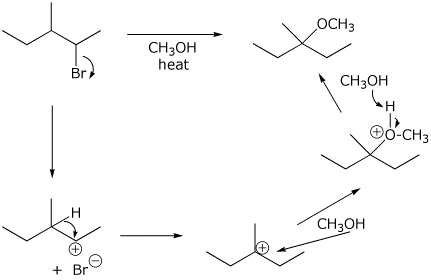
8. (15 points) Write a complete mechanism for the reaction shown below.
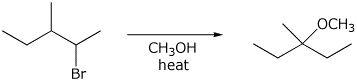
Show all steps and show electron-pushing arrows for each step.
9. (20 points) Show how to prepare the following target compounds, beginning with the indicated starting compound. Just show the reagents and conditions and the compounds formed in each reaction (no mechanisms needed). Pay attention to stereochemistry.
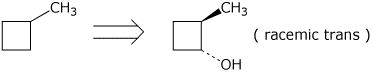

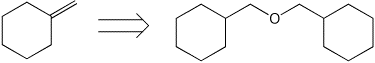

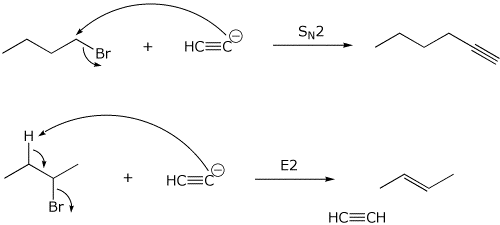
10. (10 points) Sodium acetylide ( NaC=CH ) is a good nucleophile but
also a strong base. With primary halides it gives good SN2 substitution,
but with secondary halides, it gives mostly E2 elimination.
Illustrate each of these two examples using 1-bromobutane and 2-bromobutane.
Show electron-pushing arrows for each case.
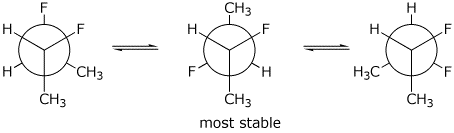
11. (15 points) Write all three conformations of meso-2,3-difluorobutane, looking down the C2-C3 bond. Predict the most stable conformation, assuming that a methyl group is larger than a fluorine atom.
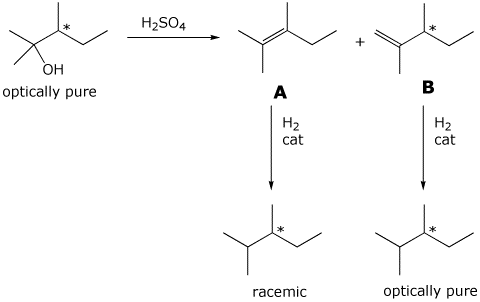
12. (15 points) A sample of optically pure 2,3-dimethyl-2-pentanol was
dehydrated with sulfuric acid to give two alkenes, A and B. A was hydrogenated
to give
optically inactive 2,3-dimethylpentane, but B was hydrogenated to give
optically pure 2,3-dimethylpentane.
Identify compounds A and B, and show each of the reactions referred to.