|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 3 Answer Key |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 3 Answer Key |
![]()
1. (15 points) Write complete names for each of the following.
a) 
(2R,3S)-2-chloro-2,3-dimethyl-1,4-butanediol
b) 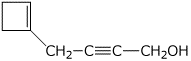
4-(1-cyclobutenyl)-2-butyn-1-ol
c) 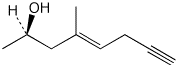
(R,E)-4-methyloct-4-en-7-yn-2-ol
2. (15 points) Write accurate structures for the following:
a) two diastereomers of 2,3-dichlorobutane
b) two enantiomers of 2,3-dichlorobutane
c) ozonolysis products from 2-pentyne
d) (R,R)-1,2-dimethylcyclopentane
e) the transition state for an SN2 reaction of I- with isobutyl bromide
3. (15 points) Arrange each of the following in
order with respect to the property indicated.
Write “MOST” under the compound with the highest value and “LEAST” under
the compound with the lowest value.
a) nucleophilicity
CH3ONa / / CH3COONa / / CH3COOH
MOST / / MIDDLE / / LEAST
b) reactivity in an SN2 mechanism
1-bromobutane / / 2-bromobutane / / 2-chlorobutane
MOST / / MIDDLE / / LEAST
c) reactivity towards methyl iodide
NaCl in EtOH / / NaCl in DMSO / / EtOH
MIDDLE / / MOST / / LEAST
d) acidity
cyclobutane / / 1-butene / / 1-butyne
LEAST / / MIDDLE / / MOST
e) ratio of substitution/elimination using NaOEt in EtOH
n-butyl bromide / / sec-butyl bromide / / tert-butyl bromide
MOST / / MIDDLE / / LEAST
4. (15 points) Complete the following reactions
by indicating the major product expected.
Show stereochemistry
if it
is specific.
If the reaction can be described as
SN1, SN2, E1, or E2, indicate that.
a) 
b) 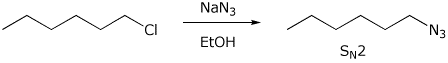
c) 
d) 
e) 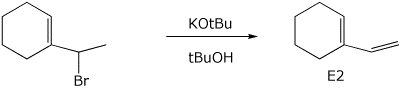
5. (15 points) Complete the following structures so as to show both enantiomers of trans-1,3-dichlorocyclohexane in both of their chair conformations. Place the Cl substituents on the positions marked with the dots.
Identify each stereocenter as R or S on EVERY structure.
Identify which conformation is the more stable one for each enantiomer.
All the four chair structures are equally stable (one axial, one equatorial).
6. (10 points) Preparation of 2,3-dibromobutane from 2-butyne can be done
by addition of one equivalent of Br2 and one equivalent of H2.
Show the addition of Br2 (anti) followed by the addition of H2 (syn), specifically illustrating the stereoisomers that would be formed at each stage.
Does it matter whether the order of addition was reversed?
The same final product will result (a racemic mixture of (R,R) and (S,S) ).
7. (15 points) Show how you could prepare butane, starting from acetylene as your ONLY source of carbon.