|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 1 |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 1 |
![]()
1. (15 points) Write complete names for each of the following.
a) 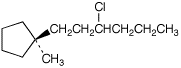
b) 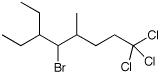
c) 
2. (15 points) Write accurate structures for the following:
a) anti 1-bromopropane
b) axial bromocyclohexane
c) 1-methylbicyclo[2.2.1]heptane
d) 1-methylspiro[3.4]octane
e) a C5 heterocycle containing one nitrogen
3. (10 points) Answer the following questions about the molecule shown below:
(3) What is the molecular formula?
(3) Indicate the number of carbons with each hybridization:
sp ____________
sp2 ____________
sp3 ____________
(4) On the structure, carefully point to ONE example each of a carbon that is
1° ____________
2° ____________
3° ____________
4° ____________
4. (15 points) Arrange each of the following in order
with respect to the property indicated.
Write “MOST” under the compound with the highest value and “LEAST” under
the compound with the lowest value.
a) acidity
b) stability
c) boiling point
d) acidity
e) heat of combustion
5. (15 points) Complete the following acid-base reactions and predict whether the equilibrium will be favored to the right or to the left.
a) ![]()
b) ![]()
c) ![]()
Of all the structures shown above (on both sides of your equations), identify which is the
d) strongest acid
e) strongest base
6. (15 points) Give the IUPAC name for the compound shown in the Newman projection below.
Is this its most stable conformation?
Write the other two staggered conformations for this compound and compare the energies of all three structures.
7.
(15 points) Complete the structure on the right so it represents the other
chair conformation of the structure on the left.
Locate all substituents
accurately. Circle all axial substituents on both structures.
Can you predict which structure is more stable? Explain.
![]()