
|
Organic
Chemistry I
|
|
Professor
Carl C. Wamser
|
Chapter
9 Notes
|
|

Alkynes
Structure
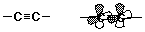
- carbon-carbon triple bond
- sp hybridization (linear)
- no cis-trans possibilities
- the two pi bonds are perpendicular
- high electron density
(usually more reactive than alkenes)
Nomenclature
- -yne infix (with number)
- rules similar to alkenes
- with both -enes and -ynes, suffix is -enyne and numbering is from the
end closer to a multiple bond
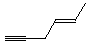
(E)-4-hexen-1-yne
- common names: alkyl acetylene or dialkyl acetylene
Properties
- acidity
sp C-H bonds are relatively acidic
alkanes (sp3 C-H), pKa ~ 62
alkenes (sp2 C-H), pKa ~ 45
alkynes (sp C-H), pKa ~ 26
- sp C-H bonds can be deprotonated by bases with pKa > 26
( e.g., NaNH2 but not NaOH )
- alkyne anions are called acetylides and are good nucleophiles
- alkylation
substitution of an alkyl group, using acetylide anion
SN2, so alkyl group must be CH3 or
1°
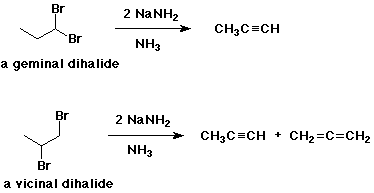
Preparations - eliminations
- NaNH2 in liquid NH3 removes
2 HX from dihalides
Reactions - Hydrogenation
- catalytic hydrogenation to an alkane
- Lindlar catalyst + H2 - forms cis-alkene
- reduction by sodium in liquid NH3 - forms trans-alkene
Addition reactions
- hydration
addition of water with acid catalysis or Hg+2 as catalyst
follows Markovnikov addition (unlike hydroboration/oxidation)
- goes through an enol intermediate

- keto-enol tautomers
carbonyl compound is generally more stable than the enol isomer
tautomers - isomers differing by the position of a hydrogen & double
bond
- halogenation
addition of two equivalents of Cl2 or Br2
- hydrohalogenation
addition of two equivalents of HCl or HBr
forms geminal dihalide
- ozonolysis
oxidation to form two equivalents of carboxylic acids - one from each half
Organic synthesis
- retrosynthetic strategy
- think backwards -
What is a reaction that could be used to make this product?
- visualize the construction of the carbon framework
What reactions make new C-C bonds?
- carry out functional group interconversions as necessary


![]()
![]()

