|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 6 Workshop |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 6 Workshop |
![]()
Alkene Reactions
1. All of the following reactions occur by analogous mechanisms. Write a single, general mechanism that explains all of these reactions. Make a table listing the electrophiles and the nucleophiles, reaction by reaction, for the first mechanistic step and also for the second mechanistic step.
RCH=CH2 + Cl2 ---> RCHClCH2Cl
RCH=CH2 + Cl2 (in H2O) ---> RCH(OH)CH2Cl
RCH=CH2 + Br2 ---> RCHBrCH2Br
RCH=CH2 + Br2 (in CH3OH) ---> RCH(OCH3)CH2Br
RCH=CH2 + HCl ---> RCHClCH3
RCH=CH2 + HBr ---> RCHBrCH3
RCH=CH2 + HI ---> RCHICH3
RCH=CH2 + H2SO4 ---> ![]()
RCH=CH2 + H2SO4 (in H2O) ---> RCH(OH)CH3
RCH=CH2 + H2SO4 (in CH3OH) ---> RCH(OCH3)CH3
RCH=CH2 + Hg(O2CCH3)2 (in H2O) ---> RCH(OH)CH2-HgO2CCH3
RCH=CH2 + Hg(O2CCF3)2 (in CH3OH) ---> RCH(OCH3)CH2-HgO2CCF3
b. The reaction of 3-methyl-2-butanol with hot H3PO4 gives three elimination products, in yields of 64%, 33% and 3%. Write a complete mechanism, which should start out analogously to part (a) above, and predict the structures of the three products. Why are substitution products formed in part (a) but are not formed in this case?
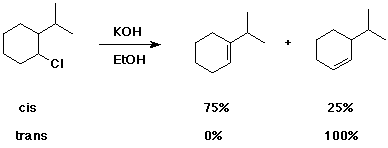
Do the same with the trans isomer. Explain the distinctive differences in the two cases.
One of the isomers reacts much faster than the other. Predict which is faster and explain the difference.
Materials adapted from:
Peer-Led Team Learning: Organic Chemistry, 1/e
Jack A. Kampmeier, University of Rochester
Pratibha Varma-Nelson, St. Xavier University
Donald Wedegaertner, University of the Pacific
Prentice-Hall, 2001, ISBN 0-13-028413-0
![]()