|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 5 Quiz |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 5 Quiz |
![]()
1. (2 points) Give complete IUPAC names for each of the following:
a) 
(E)-4-cyclopentyl-2,3-dimethyl-3-penten-1-ol
b) ![]()
(2E,4Z,6E)-4-chloro-2,4,6-octatriene
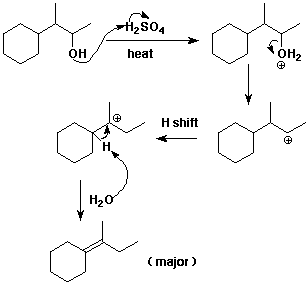
2. (3 points) Write a complete mechanism for the dehydration reaction shown below. Just show the pathway to the major product. Show all steps and use electron-pushing arrows.
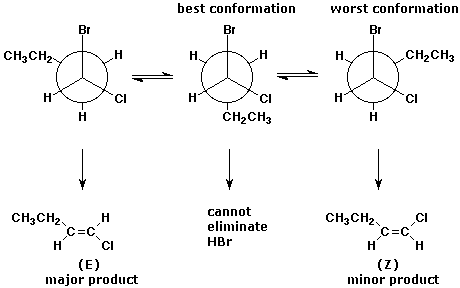
3. (5 points) Write all three conformations of 1-bromo-1-chlorobutane,
using Newman diagrams looking down the C1-C2 bond.
Bromide is a much better
leaving group than chloride, and the products are (E) and (Z)-1-chloro-1-butene.
Illustrate which conformation(s) give each product in an E2 elimination,
and predict the major product.
![]()