|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 5 HW Answers |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Chapter 5 HW Answers |
![]()
Carey, pages 223 - 229:
Problems 5.22 - 5.48
1. Write IUPAC names for each of the following, including designation of stereochemistry where needed.
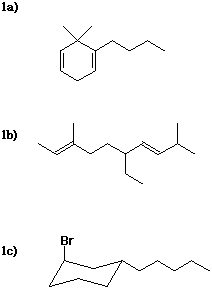
1a) 1-butyl-6,6-dimethyl-1,4-cyclohexadiene
1b) (2E,7E)-6-ethyl-3,9-dimethyl-2,7-decadiene
1c) trans-1-bromo-3-pentylcyclohexane
2. There are four isomeric alkenes of formula C4H8 . There are four isomeric alcohols of formula C4H10O . There are four isomeric alkyl bromides of formula C4H9Br .

For each of the four alcohols, predict the alkene product(s), including the expected major product, from an acid-catalyzed dehydration (E1) reaction.
For E1 dehydration reactions of the four alcohols:
E --> C (major) + B + A
F --> C (major) + B + A
G --> D
H --> D
For each of the four alkyl bromides, predict the alkene product(s), including the expected major product, from a base-promoted dehydrohalogenation (E2) reaction.
For E2 dehydrohalogenation reactions of the four alkyl bromides:
I --> A
J --> C (major) + B + A
K --> D
L --> D
For each of the four alkenes, select the best synthetic route to make that alkene, starting from any of the available alcohols or alkyl halides.
For good syntheses of the four alkenes:
A can only be made from I
B can only be isolated as a minor product from E, F, or J
C can be made as the major product from E, F, or J
D can be made from G, H, K, or L
![]()