|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 3 Answer Key |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 3 Answer Key |
![]()
1. (15 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 
(2S,3R)-2,3,4-trimethyl-1,2-pentanediol
b) 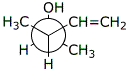
(2S,3R)-3-methyl-4-penten-2-ol
c) 
(1R,3S)-1-chloro-1-fluoro-3-methylcyclohexane
2. (15 points) Identify the relationship between the pairs of compounds shown below using the following codes:
I - identical, C - constitutional isomers, D - diastereomers, E - enantiomers,
F - conformational isomers, O - other
a) 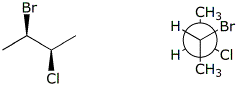 I
I
b)  D
D
c) 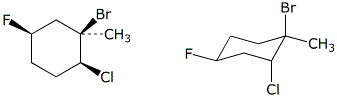 C
C
d) 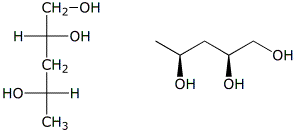 D
D
e)  E
E
3. (25 points) Complete each of the following reactions by adding the
expected major product.
Describe the stereochemistry of the product and describe the mechanism.
a) 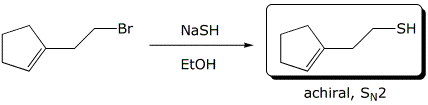
b) 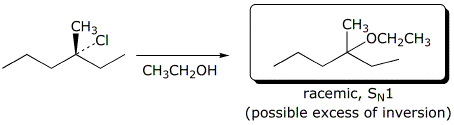
c) 
d) 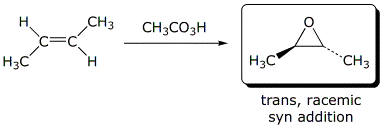
e) 
4. (20 points)
(10) Write a complete SN2 mechanism for the reaction of (R)-2-pentanol with
HI.
Show all steps and use electron-pushing arrows.
(10) Write a complete SN1 mechanism for the reaction of 2-methyl-2-pentanol
with HI.
Show all steps and use electron-pushing arrows.
5. (25 points) (R)-1,2-Pentanediol reacts with one equivalent of HBr to give a mixture of both (R)- and (S)-2-bromo-1-pentanol.
a) (6) Write out the structures of the reactants and products described.
b) (6) Explain why only one of the two alcohols reacts. Why that one?
The reaction proceeds by an SN1 mechanism and 2° is much more reactive than 1°.
c)
(6) Assume that pure (R)-2-bromo-1-pentanol has a specific rotation of
- 50°.
If the observed product is calculated to have a specific
rotation of +10°, what can you conclude about the product mixture:
optical purity = 20%
enantiomeric excess = 20%
composition of (R) and (S) = 60% (S) & 40% (R)
d) (7) From your conclusion above, explain why that particular enantiomer is favored in the reaction. Clearly show the key step in which the preference is expressed.
The nucleophile mainly comes in from the side opposite the leaving group.
![]()