|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 1 Answer Key |
![]()
|
|
Organic Chemistry I |
|
Professor Carl C. Wamser |
||
Exam 1 Answer Key |
![]()
1. (15 points) Write complete names for each of the following.
a) 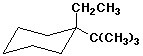
1-tert-butyl-1-ethylcyclohexane
1-ethyl-1-(1,1-dimethylethyl)cyclohexane
b) 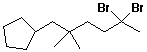
5,5-dibromo-1-cyclopentyl-2,2-dimethylhexane
c) 
6-bromo-1-chloro-6-ethyl-7,8-dimethylnonane
2. (15 points) Write accurate structures for the following:
a) bicyclo[4.2.0]octane
b) spiro[3.5]nonane
c) the most stable conformation of trans-1,3-dimethylcyclohexane
d) the lowest boiling isomer of C5H12
e) the best Lewis structure for CH2NOH
3. (15 points) Arrange each of the
following in order with respect to the property indicated.
Write “MOST” under the compound with the highest value and “LEAST” under
the compound with the lowest value.
a) stability
cis-1,2-dimethylcyclopropane / / trans-1,2-dimethylcyclopropane / / cyclopentane
LEAST / / MIDDLE / / MOST
b) heats of combustion
butane / / 2-methylbutane / / 2,3-dimethylbutane
LEAST / / MIDDLE / / MOST
c) C-C-C bond angle
cyclopropane / / cyclobutane / / cyclopentane
LEAST / / MIDDLE / / MOST
d) number of tertiary carbons
2-methylbutane / / 2,2-dimethylbutane / / 2,3-dimethylbutane
MIDDLE / / LEAST / / MOST
e) acidity
CH4 / / NH3 / / H2O
LEAST / / MIDDLE / / MOST
4a. (10 points) What is the molecular formula for the compound shown below?
Label each carbon atom with its hybridization:
* for sp3
circle each sp2
put a box around each spC12 H13 Cl O
4b. (10 points) Write an acid-base equilibrium reaction for the expected reaction when acetic acid (pKa = 4.7) is dissolved in water. Do the same for acetic acid dissolved in ammonia.
Selected pKa values are: H3O+ ( -1.7 ) , H2O ( 15.7 ) , NH4+ ( 9.3 ) , NH3 ( 36 )
Predict whether the equilibrium reactions are more favored to the right or to the left.
CH3COOH + H2O <===> CH3COO- + H3O+ (favored to the left)
CH3COOH + NH3 <===> CH3COO- + NH4+ (favored to the right)
5. (20 points) Write all the staggered conformations of 1-chloro-3,3-dimethylbutane, looking down the C1-C2 bond. Predict the most stable form.
6. (15 points) Write both chair conformations of trans-1-fluoro-3-methylcyclohexane .
The ΔG value for fluorocyclohexane favors equatorial F by 1.0 kJ/mol.
The ΔG value for methylcyclohexane favors equatorial CH3 by
7.3 kJ/mol.
Predict the more stable form.
![]()