Organic Chemistry |
||
Professor Carl C. Wamser |
||
Chem 334 - Fall 2003 |
Homework 1 Answers |
![]()
Organic Chemistry |
||
Professor Carl C. Wamser |
||
Chem 334 - Fall 2003 |
Homework 1 Answers |
![]()
Carey, pages 50 - 56:
Problems 1.33 - 1.40, 1.43 - 1.50, 1.55 - 1.65, 1.66, 1.68
1. Write good Lewis structures for the compounds listed below. The structures are written in the standard abbreviated forms, which give some indication about the order in which atoms are bonded.
Indicate the hybridization of each carbon atom and describe the expected geometry. If there are additional contributing resonance forms, show them and indicate whether they contribute equally, or if not, which is the preferred resonance form.
a) CH3OOH
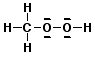
C - sp3
- molecular geometry = tetrahedral
- electron geometry = tetrahedral
each O - sp3
- molecular geometry = bent
- electron geometry = tetrahedral
b) HCOO-
both resonance forms contribute equally
C - sp2
- molecular geometry = trigonal planar
- electron geometry = trigonal planar
c) HCNO
better resonance form
C - sp
- molecular geometry = linear
- electron geometry = linear
N - sp
- molecular geometry = linear
- electron geometry = linear
d) CH3CNH+
better resonance form
C(H3) - sp3
- molecular geometry = tetrahedral
- electron geometry = tetrahedral
C - sp
- molecular geometry = linear
- electron geometry = linear
N - sp
- molecular geometry = linear
- electron geometry = linear
e) CH2OH+
better resonance form
C - sp2
- molecular geometry = trigonal planar
- electron geometry = trigonal planar
O - sp2
- molecular geometry = bent
- electron geometry = trigonal planar
![]()