Organic Chemistry |
||
Professor Carl C. Wamser |
||
Chem 334 - Fall 2003 |
FINAL EXAM |
![]()
Organic Chemistry |
||
Professor Carl C. Wamser |
||
Chem 334 - Fall 2003 |
FINAL EXAM |
![]()
1. (25 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) ![]()
b) 
c) 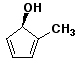
d) 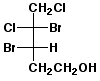
e) 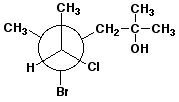
2. (15 points) Arrange each of the following in order with respect to the property indicated. Write “MOST” under the compound with the highest value and “LEAST” under the compound with the lowest value ( or “EQUAL” if appropriate).
a) acidity
methanol / ethanol / water
b) basicity
water / ammonia / sodium fluoride
c) stability
1-hexene / cis-2-hexene / trans-2-hexene
d) stability
isobutyl cation / sec-butyl cation / tert-butyl cation
e) reactivity towards cyclohexene
fluorine / chlorine / bromine
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 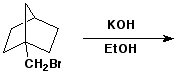
b) ![]()
c) 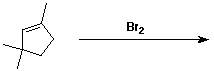
d) ![]()
e) 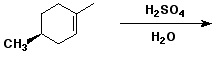
a) 
b) ![]()
c) 
d&e) 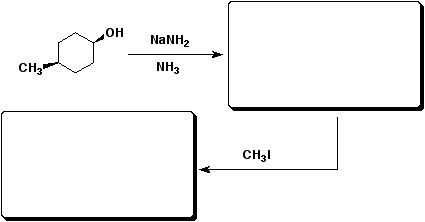
5. (15 points) Examine the pairs of structures below and identify their relationship to one another, using the letter codes below:
A - the identical structure
B - constitutional isomers
C - conformational isomers
D - diastereomers
E - enantiomers
F - none of the above
a) 
b) 
c) 
d) 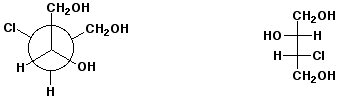
e) 
6. (16 points) Identify the hybridization of every carbon in each
of the following structures.
Rewrite each structure to show accurate three-dimensionality about
each carbon.
(Place every carbon in the plane of the paper)
a) ![]()
b) ![]()
7a). (15 points) Complete each acid-base reaction and predict the preferred direction of the equilibrium. Use “RIGHT” or “LEFT”.
i) ![]()
ii) ![]()
iii) ![]()
7b). (15 points) Calculate ΔH for each of the following reactions. Use units of kcal/mol.
i) ![]()
ii) ![]()
iii) ![]()
8a. (16 points) Consider the free radical chlorination of methylcyclopropane. Write names and structures for all possible products. For any chiral products, just write one enantiomer.
8b. (10 points) Select any one product from above and write the propagation steps in the chain mechanism that would lead to that product.
9a. (11 points) Write both chair conformations of (1S,3R)-1,3-dimethylcyclohexanol.
Predict the more stable form.
9b. (20 points) Write a complete mechanism for the reaction of (1S,3R)-1,3-dimethylcyclohexanol with HBr (substitution only). Show all steps and use electron pushing arrows. Show the expected stereochemistry of the products.
10. (12
points) Write a sequence of reactions that could convert cyclopentane
to trans-2-chlorocyclopentanol.
Show each reaction with reagents and conditions and the product
after each reaction step. Mechanisms are not needed.
![]()