Organic Chemistry |
||
Professor Carl C. Wamser |
||
Chem 334 - Fall 2003 |
EXAM 3 Answers |
![]()
Organic Chemistry |
||
Professor Carl C. Wamser |
||
Chem 334 - Fall 2003 |
EXAM 3 Answers |
![]()
1. (15 points) Write complete names for each of the following, including stereochemistry if it is specifically shown.
a) 
(2E,4S,6Z)-4-chloro-2,6-octadien-1-ol
b) 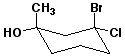
(1S,3S)-3-bromo-3-chloro-1-methylcyclohexanol
c) 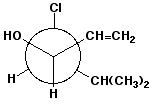
(3R,4S)-4-chloro-5-methyl-1-hexen-3-ol
2. (15 points) Arrange each of the following in order with respect to the property indicated. Write “MOST” under the compound with the highest value and “LEAST” under the compound with the lowest value ( or “EQUAL” if appropriate).
a) nucleophilicity
H2O / NaOH / NaSH
LEAST / MIDDLE / MOST
b) nucleophilicity
NaI / NaBr / I2MOST / MIDDLE / LEAST
c) optical rotation (absolute value)
(R)-2-chloropentane / (S)-2-chloropentane / racemic-2-chloropentane
EQUAL / EQUAL / LEAST
d) rate of hydrolysis in neutral water
LEAST / MIDDLE / MOST
e) rate of reaction with NaN3 in DMSO
LEAST / MIDDLE / MOST
3. (15 points) Complete each of the following reactions by adding the missing part: either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it is specific.
a) 
b) 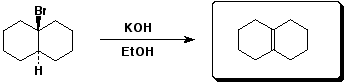
c) 
d&e) 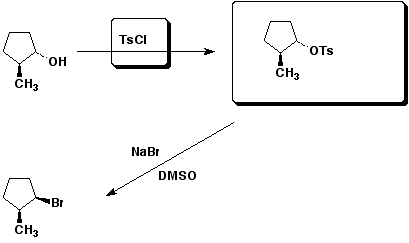
4a. (10 points) Complete the reaction shown below, initially ignoring stereochemistry.
If the original compound is the (R) stereoisomer, the product is expected to be:
(R) / (S) / racemic / can’t tell / other
If the original compound has an optical rotation that is (+), the product is expected to be:
(+) / (-) / optically inactive / can’t tell / other
4b. (10 points) Complete the reaction shown below, initially ignoring stereochemistry.
If the original compound is the (R) stereoisomer, the product is expected to be:
(R) / (S) / racemic / can’t tell / other
If the original compound has an optical rotation that is (+), the product is expected to be:
(+) / (-) / optically inactive / can’t tell / other
5. (15 points) Examine the pairs of structures below and identify their relationship to one another, using the letter codes below:
A - the identical structure
B - constitutional isomers
C - conformational isomers
D - diastereomers
E - enantiomers
F - none of the above
a)  D
D
b) 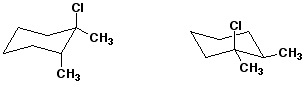 D
D
c) ![]() B
B
d) 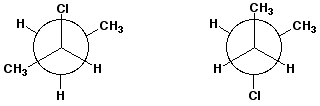 E
E
e)  E
E
6a. (12 points) Write a complete mechanism for the solvolysis (substitution only) reaction shown below. Show all steps and electron pushing arrows. Do not consider any rearrangements.
6b. (8 points) In order to help relieve the ring strain, a cation rearrangement can occur. Write a clear electron-pushing mechanism that illustrates how a larger ring may be formed and show the final product that would result.
![]()