Chem 334 - Fall 2001
Organic Chemistry I
Dr. Carl C. Wamser

1. (2 points) Complete the following reaction by adding the missing part: the starting compound, the necessary reagents and conditions, or the final major product. Include stereochemistry if it is specific. Identify the most likely mechanism by name (e.g., SN2)
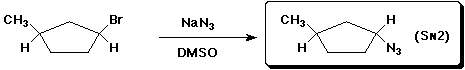
2. (2 points) Arrange the four isomeric butyl bromides (C4H9Br) in order of their expected reactivity in an SN2 mechanism.
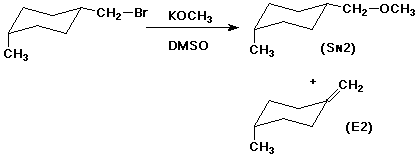
3. (2 points) The reaction below produces both SN2 and E2 products. Write the structures of the expected products from each mechanism.
4. (4 points) The reaction below follows an SN1 mechanism but gives mainly product with RETAINED stereochemistry. Write a complete mechanism and explain the preference for the observed major product.
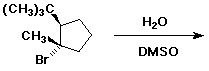

Attack of the nucleophile (H2O) on the carbocation is not
the same from top or bottom because of the adjacent stereocenter.
The nucleophile will mainly avoid the bulky tert-butyl group and
attack from the opposite side (which happens to be the side that
the Br left from).
Note that the same product mixture would be expected starting
from the other stereoisomer (in which Br was originally cis to
tert-butyl).