Chem 334 - Fall 2001
Organic Chemistry I
Dr. Carl C. Wamser

1. (3 points) For each of the pairs of compounds below, indicate the relationship between the two structures using one of the letters below.
A - - they are the same compound
B - - they are resonance forms
C - - they are constitutional isomers
a)  C
C
b)  A
A
c)  C
C
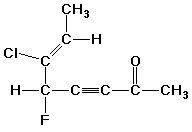
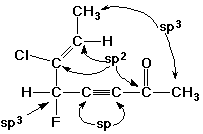
2. (2 points) Identify the hybridization of every carbon in the structure below.
3. (5 points) The compound CH2NO-
has two good Lewis structures that are the two major contributing
resonance forms. Write them both, including accurate geometry
and all lone pairs, and predict which is the more stable form.
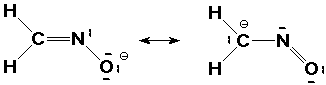
The negative charge is better on oxygen, because it is more electronegative.