
Chem 334 - Fall 2001
Organic Chemistry I
Dr. Carl C. Wamser

1. (20 points) Write complete names for each of the following, including stereochemistry if it is specifically shown:
a) 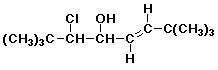
(E)-3-chloro-2,2,7,7-tetramethyl-5-octen-4-ol
trans is also OK
b) 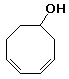
3,5-cyclooctadienol
c) ![]()
trans-4-vinylcyclohexanol
trans-4-ethenylcyclohexanol
d) 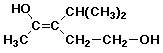
(Z)-3-isopropyl-3-penten-1,4-diol
3-(1-methyethyl) is also OK
2. (15 points) Arrange the following sets of compounds in
order with respect to the property indicated.
Write "MOST" and "LEAST" under the compounds
with the highest and lowest values of the property.
a) stability
![]()
LEAST / / MOST / / MIDDLE
b) stability
![]()
MIDDLE / / MOST / / LEAST
c) rate of dehydration
![]()
MOST / / MIDDLE / / LEAST
d) rate of bromine addition

MOST / / MIDDLE / / LEAST
e) rate of SN2 substitution by HBr
![]()
LEAST / / MOST / / MIDDLE
3. (15 points) Complete each of the following reactions by adding the missing part - either the starting compound, the necessary reagents and conditions, or the final major product. Show stereochemistry if it would be specific.
a) 
b) 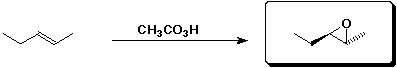
c) 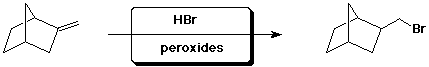
d) 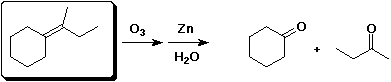
e) 
4. (15 points) Heats of combustion for four isomers are
indicated below.
Arrange the four compounds in order of stability.
Heats of combustion
cyclobutane 2721 kJ/mol LEAST STABLE
1-butene 2717 kJ/mol
cis-2-butene 2710 kJ/mol
trans-2-butene 2706 kJ/mol MOST STABLE
The heat of hydrogenation for trans-2-butene is 115 kJ/mol. Calculate what the other three values should be.
Heats of hydrogenation
cyclobutane 130 kJ/mol
1-butene 126 kJ/mol
cis-2-butene 119 kJ/mol
trans-2-butene 115 kJ/mol
Using C4H8 to represent any of the four isomers, write the balanced reaction that corresponds to the heat of combustion.
C4H8 + 6 O2 ------> 4 CO2 + 4 H2O
Using C4H8 to represent any of the four isomers, write the balanced reaction that corresponds to the heat of hydrogenation.
C4H8 + H2 ------> C4H10
Heating cyclobutane can lead to a ring opening to form 1-butene.
Calculate the expected delta H for this reaction.
Is it exothermic or endothermic?
delta H = - 4 kJ/mol (exothermic)
5. (10 points) Free radical halogenation of ethylcyclopropane
can lead to five possible monochlorination products.
Write structures for all of them.
6. (10 points) Complete the following synthetic sequence by adding the missing parts.
7. (15 points) Write a complete mechanism for the SN1
substitution reaction shown below.
Write all steps and show electron-pushing arrows.
Elimination products may also occur in this reaction. Show the structure of any elimination products expected.