
Chapter 4 Notes: Alcohols and Alkyl Halides

Functional groups
alkyl halide: R-X (X = halogen atom)
alcohol: R-O-H (hydroxyl group)
Functional Group Classification
- based on the carbon the X or OH group is attached to:
1° , 2° , 3°
- ethyl alcohol
CH3CH2OH (1°)
- isopropyl chloride
(CH3)2CHCl (2°)
- t-butyl alcohol
(CH3)3COH (3°)
Alkyl Halide Nomenclature
- halogen substituents are indicated by the prefixes fluoro-,
chloro-, bromo-, and iodo- and listed in alphabetical order with
other substituents
- common names - name the alkyl group followed by the name
of the halide
e.g., methyl chloride or chloromethane
- several polyhaloalkanes are common solvents and are generally
referred to by their common names
e.g., chloroform or trichloromethane, Freon 12 or dichlorodifluoromethane
- hydrocarbons in which all hydrogens are replaced by halogens
are commonly named as perhaloalkanes
e.g., perfluoroethane or 1,1,1,2,2,2-hexafluoroethane
Alcohol Nomenclature
- common name: alkyl alcohol
- IUPAC: alkanol
- OH group takes priority over substituents
- it must be in the parent chain
- the direction of numbering gives it the lower possible number
(regardless of substituents)
- use -ol suffix with number designation
- name other substituents as prefixes as usual
- more than one alcohol named as a -diol, -triol, etc.
Alcohol Examples
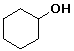
cyclohexyl alcohol or cyclohexanol

trans-4-methylcyclohexanol
Structure of Alcohols
- sp3 O with two covalent bonds and two lone pairs
- The bond angle about oxygen is about 105°, explained
as greater repulsions of the lone pairs towards the bonding pairs
Boiling Points of Alkyl Halides
- among constitutional isomers, branched isomers have a more
compact shape, decreased area of contact, decreased van der Waals
attractive forces between neighbors, and lower boiling points
- 1-bromobutane (n-butyl bromide) bp = 100°
- 2-bromo-2-methylpropane (t-butyl bromide) bp = 72°
- for an alkane and an alkyl halide of comparable size and
shape, the alkyl halide has the higher boiling point
the difference is due almost entirely to the greater polarizability
of the three unshared pairs of electrons on halogen compared
with the polarizability of shared electron pairs of the hydrocarbon
- ethane bp = -89°
- bromomethane bp = 4°
- boiling points of alkyl fluorides are lower than those of
hydrocarbons of comparable molecular weight
the difference is due to the small size of fluorine, the tightness
with which its electrons are held, and their particularly low
polarizability
- 2-methylpropane bp = - 1°
- 2-fluoropropane bp = - 11°
Polarity of Alkyl Halides
- dipole moment of RX depends on:
the sizes of the partial charges,
the distance between them, and
the polarizability of the unshared electrons on halogen
- dipole moments of all the haloalkanes are comparable
Hydrogen Bonding
- attraction between the positive end of one dipole (an H bonded
to F, O, or N - atoms of high electronegativity) and the negative
end of a dipole, usually a lone pair on F, O, or N
- in alcohols, O lone pairs interact with polar H bonds
- covalent O-H bond strength ~ 100 kcal/mole
- O...H (H-bond) strength ~ 5 kcal/mole
Effects of H-Bonding
- alcohols have higher boiling points than alkanes (nonpolar)
or alkyl halides (polar, but no H-bonds)
- ethers are polar but have no H-bonds
(pentane and diethyl ether both boil at about 35°, but 1-butanol
has a bp of 117°)
- H-bonds hold together the strands of DNA ("Velcro"
effect)
- H-bonding increases water solubility
Review of Acid-Base Reactions
Bronsted-Lowry Acid/Base
- acid - donates H+
- base - accepts H+
NH3 + H2O <==>
NH4+ + OH-
base + acid <==> acid + base
- note conjugate acid-base pairs
(differ by H+)
Acidity Constant (Ka)
usually simplified to
HA <==> H+ + A-
Acid Strength (pKa)
- stronger acids have higher Ka
for HCl, Ka = 10E7
for CH3COOH, Ka = 10E-5
(acetic acid, found in vinegar)
- pKa = - log Ka
- stronger acids have a lower pKa
for HCl, pKa = -7
for CH3COOH, pKa = 5
pH and pKa
- Ka = [H+][A-]/[HA]
- pKa = pH - log([A-]/[HA])
- for pH = pKa, [A- ] = [HA]
- for pH < pKa, HA predominates
- for pH > pKa, A- predominates
e.g., for acetic acid at pH = 7
[CH3COO-] > [CH3COOH]
Structural Effects on Acid Strength
- electronegativity
HF > H2O > NH3
> CH4
- weaker bond to H
HI > HBr > HCl > HF
- inductive effects - electron withdrawal
H2SO4 > H2SO3
Cl-CH2-COOH > CH3-COOH
- hybridization with greater s-character
sp C-H > sp2 C-H > sp3 C-H
- delocalization
RCOOH > ROH
Acid-Base Reactions
CH3COOH + OH- <==> CH3COO-
+ H2O
acid (pKa = 5) + base <==> base + acid (pKa = 15.7)
reaction favored for stronger acid
reaction favored to the right
Lewis Acids
- acid - accepts an electron pair
- base - donates an electron pair
(making a new covalent bond)
Acid-Base Reactions of Alcohols
- remember analogy with water
- reactions as bases:
H2O + H+ <==> H3O+
ROH + H+ <==> ROH2+ (an oxonium
ion)
- reactions as acids:
H2O + B- <==> B-H + OH-
ROH + B- <==> B-H + RO- (an alkoxide
ion)
Mechanism of Proton Transfer - Potential energy diagram
- potential energy (delta H or delta G) vs reaction progress
(or reaction coordinate)
- features of a typical potential energy diagram: transition
state, delta H, Ea
Acidity of Alcohols
- alcohols about as acidic as water
MeOH more acidic, EtOH less acidic
3° alcohols much weaker acids
- pKa values: 3° > 2° > 1° > MeOH
18 , 17, 16, 15.5 (compare H2O: pKa =
15.7)
- tBuOH + NaOH ---> unfavorable
Substitution Reactions with HX - SN1 Mechanism
- halide substitution by a three-step (SN1)
mechanism
tBuOH + HBr --> tBuOH2+ --> tBu+
--> tBuBr
- acid-base reaction sets up H2O as
a good leaving group
- carbocation (3°) intermediate
- Br- as a nucleophile
- potential energy diagram
- rate-determining step is formation of the carbocation
Carbocations
- structure: trivalent sp2 C with only 6 electrons (electron-deficient)
- stability order: favored by electron donation by substituents
3° > 2° > 1°
Reactivity
- alcohol reactivity parallels carbocation stability: 3°
> 2° > 1°
- more stable intermediate (carbocation) implies a lower Ea
(more stable transition state)
Hammond's Postulate
- What does a transition state look like?
- a transition state looks like something between reactants
and products
but closer in structure to whichever it is closer in energy to
- Hammond's Postulate: the structure of the transition
state
for an exothermic reaction looks more like the reactants of that
step
for an endothermic reaction looks more like the products of that
step
- for the SN1 substitution, the rate-determining step is formation
of the carbocation (endothermic)
- transition states look mainly like a carbocation (and are
more stable if the carbocation is more stable)
Substitution Reactions with HX - SN2 Mechanism
- 1° and methyl alcohols don't form stable carbocations,
don't do SN1 well
MeOH + HBr --> MeOH2+ Br- --> MeBr
+ H2O
concerted displacement of H2O by Br- (bimolecular)
Halogen Substitution with Other Reagents
- PBr3 + alcohol gives alkyl bromide,
with less rearrangement than with HBr
- SOCl2 + alcohol gives alkyl chloride,
also with less rearrangement
Halogenation of Alkanes

Free Radicals
- radical: any chemical species that contains one or more unpaired
electrons
- radicals are formed by homolytic cleavage of a bond
- a barbed curved (fishhook) arrow is used to show the change
in position of a single electron
- the order of stability of alkyl radicals is 3° > 2°
> 1° > CH3
- radical initiators like peroxides (ROOR) have weak bonds,
eaily broken
Mechanism - Chain initiation
- a step in a radical chain reaction characterized by formation
of radicals from nonradical compounds
Mechanism - Chain propagation
- a step in a radical chain reaction characterized by reaction
of a radical and a molecule to form a new radical
- Cl. + CH3CH3 --> CH3CH2. + HCl
- CH3CH2. + Cl2 --> CH3CH2Cl + Cl.
- notice that the radicals recycle
- chain length, n: the number of times the cycle of chain propagation
steps repeats in a chain reaction
Mechanism - Chain termination
- a step in a radical chain reaction that involves destruction
of radicals
- 2 Cl. --> Cl2
- CH3CH2. + Cl. --> CH3CH2Cl
- 2 CH3CH2. --> CH3CH2CH2CH3
Energetics
- using BDE data, calculate the heat of reaction, delta H°,
for the halogenation of an alkane
- delta H = sum of bonds broken - sum of bonds made
Halogenation of Larger Alkanes
- regioselectivity of 2° hydrogen over a 1° hydrogen
is high for bromination
- propane + Br2 --> 1-bromopropane (8%) + 2-bromopropane
(92%)
- regioselectivity is not as high for chlorination
- propane + Cl2 --> 1-chloropropane (43%) + 2-chloropropane
(57%)
- regioselectivity is 3° > 2° > 1°
for bromination - approximately 1600:80:1
for chlorination - approximately 5:4:1
- predict products based on regioselectivity (energetic factor)
x statistical factor (number of H's)
- example: draw all monobromination products for isobutane
and predict the % of each for a given reaction
Regioselectivity
- the regioselectivity of chlorination and bromination
can be accounted for in terms of the
relative stabilities of alkyl radicals (3° > 2° >
1° > methyl)
- how do we account for the greater regioselectivity of
bromination (1600:80:1) compared with chlorination (5:4:1)?
Hammond's Postulate
- in halogenation of an alkane, the rate-limiting step is hydrogen
abstraction
this step is exothermic for chlorination and endothermic for
bromination
- because hydrogen abstraction for chlorination is exothermic,
the transition state resembles the alkane and a chlorine atom,
there is little radical character on carbon in the transition
state, and
regioselectivity is only slightly influenced by radical stability
- because hydrogen abstraction for bromination is endothermic,
the transition state resembles an alkyl radical and HBr,
there is significant radical character on carbon in the transition
state, and
regioselectivity is greatly influenced by radical stability.
Radical stability
- 3° > 2° > 1° > methyl
- 3° C-H bond 91 kca/mol
- 2° C-H bond 95 kca/mol
- 1° C-H bond 98 kca/mol
- regioselectivity is in the same order
- the order is like carbocation stability and for a similar
reason -
the radical center is electron-deficient and needs electron donation

![]()