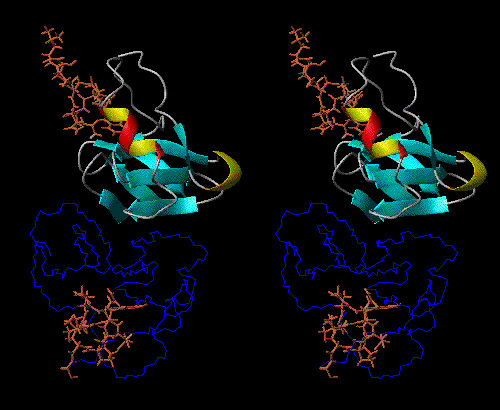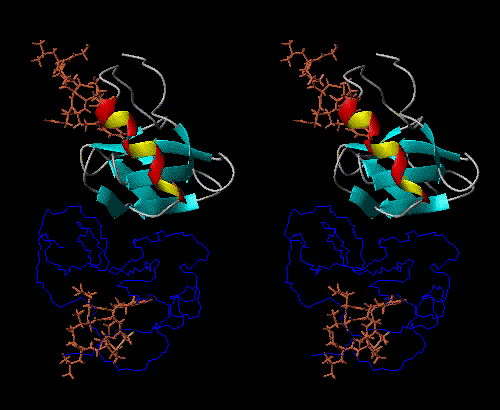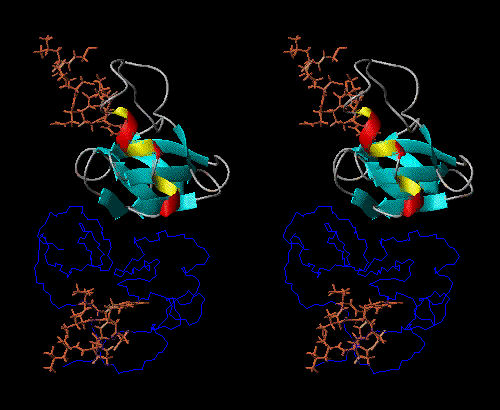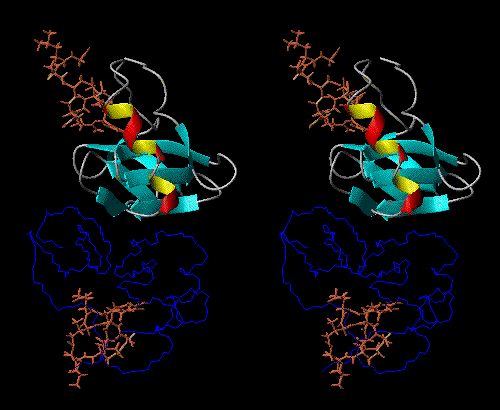
NPs dimerize in solution, with positive thermodynamic linkage between dimerization and peptide-hormone binding. In other words, the NP dimer binds to the peptide-hormone more tightly than does the monomer, and the peptide-hormone bound state dimerizes more tightly than does the unliganded state.
The peptide-hormone binding site and dimerization surface are spatially
remote from each other, so NPs can be thought of as models for allosteric
behavior, so that the monomer, unbound dimer, and bound dimer all have
different conformations (Fig 1).
Our work proposed will begin to bridge the gap in understanding between NP monomer and dimer by providing high-resolution solution-state structural data for NP. We are asking fundamental questions about the relation of NP structure and dynamics to its function.
Toward these ends, we are attempting a full assignment of NP's NMR spectrum. This will lead to a calculation of its solution-state structure. Related work includes molecular dynamics of the protein, starting with the available x-ray structures. The molecular dynamics is focused primarily on issues relating to the NMR spectroscopy.
NMR (TOCSY) spectrum of NP dimer:
Molecular Dynamics of oxytocin-NP-II dimer: