![]()
![]()
1. a. Discuss the similarities and differences in the structures and reactivities of the double bonds of 2-methylpropene and 2-propanone.
b. With part a in mind, account for the following observations. Identify the nucleophile and the electrophile for the first step of each reaction.
2. Write detailed electron-pushing mechanisms that explain the stated observations.
a. The following reaction occurs rapidly at pH = 5 but fails at pH = 1.
b. Acetone can be labeled with 18O either acidic or basic solution. The labeling of acetone with 18O is catalyzed by both acid and base.
3. When 2,2-dimethoxypropane is mixed with n-butanol and some p-toluenesulfonic acid in benzene, the equilibrium shown below is established. The lowest boiling component of this mixture is methanol. Provide a reasonable mechanism for the reaction and suggest an experimental technique that could be used to shift the equilibrium to the right and thus make the reaction useful for synthesizing the dibutylketal. (Note: There is no water present. Therefore, the reaction does NOT proceed by hydrolysis of the ketal to acetone.)
4. Give a reasonable mechanism for each of the following reactions, clearly showing all important intermediates. Show movement of electron pairs with curved arrows.
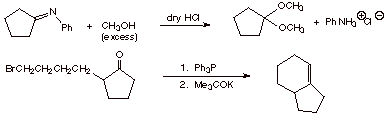
5. Pinacol rearrangement is a reaction that produces carbonyl compounds. Give a mechanism for the following reaction:
The following reaction gives only one product as shown.
What are the other two possible products? Explain the observed selectivity.
Materials adapted from:
Peer-Led Team Learning: Organic Chemistry, 1/e
Jack A. Kampmeier, University of Rochester
Pratibha Varma-Nelson, St. Xavier University
Donald Wedegaertner, University of the Pacific
Prentice-Hall, 2001, ISBN 0-13-028413-0
http://www.sci.ccny.cuny.edu/~chemwksp/OrganicChem.html