![]()
![]()
Chapter 19 Workshop Problems
Aromaticity / Molecular Orbitals
1. The following are some of the structures proposed for benzene over the years. All of them have been synthesized and are known to revert to benzene when warmed. With the help of arrows show how each of these rearranges to benzene. How would the NMR of each of these differ from that of benzene?
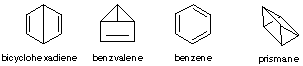
2. Provide reasonable explanation for the following observations:
a. Pyridine and pyrrole exhibit the properties of typical aromatic compounds. Pyridine is commonly used as a base; the pKa of the corresponding conjugate acid is 5.29. On the other hand, pyrrole is not basic; the pKa of the corresponding conjugate acid is -4.4.
b. Despite the ring strain, cyclopropenium ion represents one of the most stable carbocations known. Explain this stability. Predict chemical properties for cyclopropene and cyclopropenone.

c. Explain the proton chemical shifts for the following compound: 9.0 ppm and -3.0 ppm
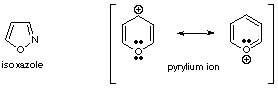
3. The compounds shown below exhibit the properties of typical aromatic systems.
a. Propose the geometry at each atom in the rings.
b. Propose hybridization schemes for each atom in the rings.
c. Specify the bonding, using lines for sigma bonds, showing overlapping
p orbitals for pi bonding, and locating the non-bonding electrons.
4. Consider the cyclooctatetraene dication, i.e., COT from which two p electrons have been removed. Provide a diagram of the energy levels of the pi molecular orbitals of the dication showing how they are occupied by the pi electrons and predict whether the system will be aromatic or antiaromatic.
5. Construct the molecular orbitals of the pi-system of benzene
from two allyl pi-systems as shown below. Place the relevant
pi molecular orbital(s) (MO's) of one of the allyl pi-systems
on the left side of the paper and the other on the right side.
Ignore the sigma framework. The molecular orbitals of benzene
should be drawn in between. The nodal properties (i.e.,
symmetry) and the relative energies of all of the orbitals should
be clearly illustrated. (Hint: You do not need to consider interactions
between molecular orbitals of largely different energies). Compare
the energies of the 4 e- and 6 e- pi-systems of benzene relative
to that of two allyl fragments.
