![]()
![]()
![]()
McMurry, pp 405 - 409:
Problems 13.18-20, 23, 26-36, 38-41, 49-52
1. Predict the general features of the H and 13C NMR spectra of the following
compounds. Use the format of question 2 below.
a) diethyl ketone

H NMR:
1-2 ppm, area 6, triplet (methyls)
2-3 ppm, area 4, quartet (methylenes)
13C NMR (proton uncoupled):
three peaks: ~30 ppm (methyls), ~50 ppm (methylenes), ~200 ppm (carbonyl)

H NMR:
1-2 ppm, area 3, triplet (methyl)
2-3 ppm, area 2, quartet (methylene)
7-8 ppm, area 4, multiplet (aromatic)
11-12 ppm, area 1, singlet (carboxylic acid)
13C NMR (proton uncoupled):
seven peaks: ~30 ppm (methyl), ~50 ppm (methylene), 120-180 ppm (4 different
aromatic carbons), ~200 ppm (carboxylic acid)

H NMR:
1-2 ppm, area 3, doublet (methyl)
2-3 ppm, area 2, triplet (methylene) (actually a doublet of doublets)
3-4 ppm, area 3, singlet (methoxy)
3-4 ppm, area 1, multiplet (methine - CH) (actually a triplet of quartets)
9-10 ppm, area 1, triplet (aldehyde)
13C NMR (proton uncoupled):
five peaks: ~30 ppm (methyl), ~50 ppm (methylene), ~70 ppm (methoxy), ~80
ppm (methine - CH), ~200 ppm (carbonyl)
2.72 ppm, area 2, quintet
4.73 ppm, area 4, triplet

11.0 ppm, area 1, singlet
7.0 ppm, area 5, multiplet
3.0 ppm, area 2, singlet
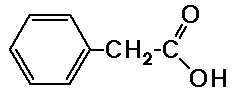
7.05 ppm, area 4, singlet
2.45 ppm, area 4, quartet
1.05 ppm, area 6, triplet

3.80 ppm, area 1, multiplet
3.50 ppm, area 2, doublet
1.12 ppm, area 3, doublet

4.82 ppm, area 2, singlet
2.22 ppm, area 4, triplet
1.65 ppm, area 4, triplet
