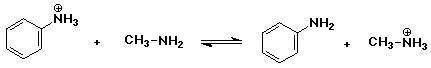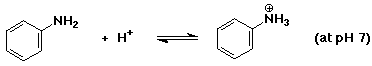Chemistry 332 - Spring 1996
Elements of Organic Chemistry II

Homework, Chapter 12 - Amines
McMurry, pp 375-377:
Problems 12.24-30, 32, 38-40, 50
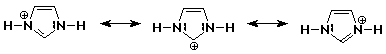
1. Write all the resonance forms for protonated imidazole. One is shown
below.
2. Predict whether the following acid-base equilibria would be more favorable
to the right or to the left. Use pKa data from your text.
a) 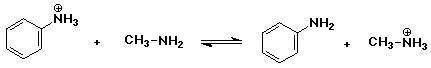
pKb = 9.37 for aniline and pKb = 3.36 for methylamine
- this means methylamine is the stronger base. The reaction will be favored
to the RIGHT.
b) 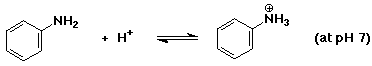
Since pKb = 9.37 for aniline, then pKa = 4.63 for
its conjugate acid. pH 7 is ABOVE the pKa value, so the basic
(neutral) form will predominate.
c) 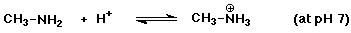
Since pKb = 3.36 for methylamine, then pKa = 10.64
for its conjugate acid. pH 7 is BELOW the pKa value, so the acidic
(ammonium ion) form will predominate.
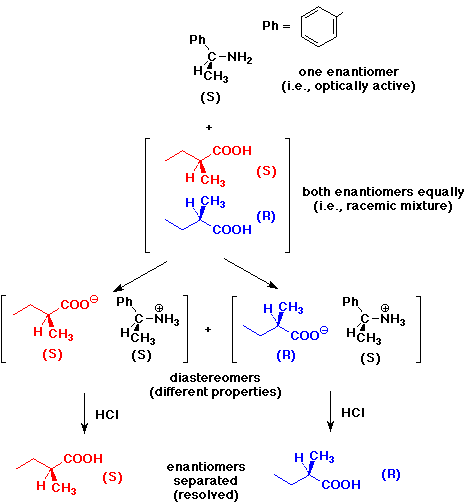
3. Given an optically active sample of 1-amino-1-phenylethane, write
a series of reactions that could be used to resolve a racemic mixture of
2-methylbutanoic acid.