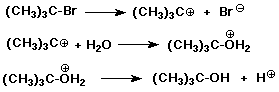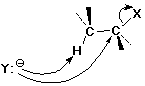Professor Carl C. Wamser

Chapter 7 - Alkyl Halides
Alkyl Halides
- Functional group: C-X bond
- X = halogen
F (fluoro)
Cl (chloro)
Br (bromo)
I (iodo)
Nomenclature
- same IUPAC rules as for other substituents (like alkyls)
- common names: alkyl halide
ethyl chloride
isopropyl iodide
benzyl bromide
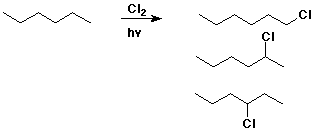
Chlorination of Alkanes
- CH4 + Cl2 --(hv)--> CH3Cl + HCl
- R-H + X2 --(hv)--> R-X + HX
- replacement of H by halogen
- initiated by light (or heat)
- works best for Cl2
- slow for Br2
- unreactive with I2
- explosive with F2
Chlorination Products
- not very useful synthetically
- usually multiple products
e.g., CH4 --> CH3Cl --> CH2Cl2 --> CHCl3 --> CCl4
Chlorination Mechanism
- radical chain mechanism
(like polymerization)
- initiation (generation of radicals)
- propagation (reaction of radicals)
products are generated in these steps
net reaction is the sum of these steps
- termination (loss of radicals)
Chlorination Mechanism
- Initiation:
Cl-Cl + hv ----> 2 Cl·
- Propagation:
Cl· + CH4 ----> CH3· + H-Cl
CH3· + Cl-Cl ----> CH3-Cl + Cl·
(repeat the propagation steps)
- Termination:
e.g., 2 CH3· ---> CH3CH3 (minor product)
Alcohols to Alkyl Halides
- R-OH + HX ----> R-X + H2O
works best for 3° alcohols
- 1° and 2° alcohols converted by:
R-OH --(SOCl2)--> R-Cl
R-OH --(PBr3)--> R-Br
Grignard Reagents
- R-X + Mg ----> R-Mg-X
- works for almost all R groups
- works for X = Cl, Br, I
- makes a nucleophilic C
- reacts with electrophiles
- RMgX + H2O ----> RH
(many more reactions later)
Nucleophilic Substitution
- R-X + Nu:- ----> R-Nu + X:-
- replacement of X- (a leaving group)
- X- often halide
- wide variety of nucleophiles
- very versatile synthetic reaction
Recognizing Nucleophiles
- must have a pair of electrons
- often have a negative charge
- often are basic
- larger halides are better nucleophiles
- I- > Br- > Cl- > F-
The SN2 Mechanism
- concerted (single step)
- involves backside attack
(simultaneous bond-making and bond-breaking)
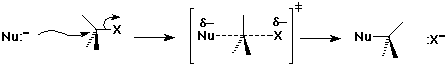
SN2 Mechanism - Evidence
- reaction rate proportional to concentration of both reactants
Rate = k [RX] [Nu]
- kinetics are second-order
- mechanism is bimolecular
SN2 Mechanism - Evidence
- stereochemistry is inverted
- indicates backside attack

SN2 Mechanism - R Groups
- bulky R groups react much slower
- reactivity order in SN2:
CH3 > 1° > 2° > 3°
- steric hindrance to attack by the nucleophile slows the rate
n-Bu > i-Bu > s-Bu > t-Bu
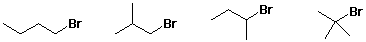
SN2 Mechanism - X Groups
- larger leaving groups react faster
I- > Br- > Cl- >> F-
- poor leaving groups
(unstable anions, strong bases)
OH- , RO- , NH2-
SN1 Mechanism
- two-step mechanism
first X- leaves, then Nu- bonds
- carbocation intermediate
R-X ----> R+ + X-
R+ + Nu- ----> R-Nu
SN1 Mechanism - Evidence
- reaction rate proportional to concentration of RX reactant,
but independent of concentration of Nu
Rate = k [RX]
- kinetics are first-order
- mechanism is unimolecular
SN1 Mechanism - Evidence
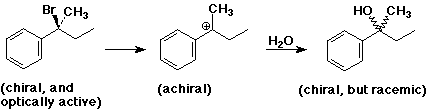
- stereochemistry is scrambled
- chiral reactant gives racemic product
- carbocation intermediate is achiral
SN1 Mechanism - R Groups
- R groups that make more stable carbocations react faster
3° > 2° > 1° > CH3
- tertiary RX react by SN1
- CH3 and primary RX react by SN2
- secondary RX react either way
SN1 Mechanism - X Groups
- same effects as for SN2
I- > Br- > Cl- >> F-
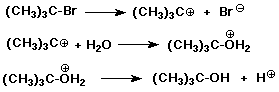
Elimination Reactions
- another common reaction of alkyl halides,
usually in competition with substitution
- base removes H+ as X- leaves
base attacks H
(nucleophile attacks C)
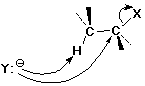
E2 Elimination Mechanism
- concerted (single-step)
- the H and X eliminated must be aligned anti to one another
- the breaking sigma bonds merge into overlapping p orbitals for the
pi bond
E2 Stereochemistry
- anti periplanar arrangement of H and X may lead to specific products
E1 Elimination Mechanism
- two-step mechanism
first X- leaves, then H+ leaves
- carbocation intermediate
- same first step as SN1
Substitution or Elimination?
- substitution: nucleophile attacks C
either attacks RX [SN2] or R+ [SN1]
- elimination: base attacks H
either attacks RX [E2] or R+ [E1]
Nucleophile or Base?
- most nucleophiles are also bases
(and vice versa)
- to favor elimination:
use a strong, hindered base
e.g., KOtBu
- to favor substitution:
use a small, unhindered nucleophile
Reactivity Patterns
- CH3X - can only do SN2
- primary (1°) RCH2X :
SN2 works well, E2 with KOtBu
SN1 and E1 don't work
- secondary (2°) R2CHX :
SN2 works with a good nucleophile
E2 works with KOtBu
SN1 and E1 occur without strong base or nucleophile
- tertiary (3°) R3CX :
SN1 works well with a good nucleophile
E1 often competes with SN1
E2 works well with KOtBu
Biological Substitutions
![]()
![]()
![]()

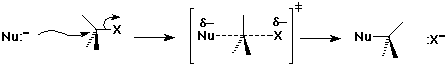

![]()