![]()
![]()
![]()
1. (15 points) Write a complete IUPAC name for each of the following compounds, including designation of stereochemistry if it is specifically shown:
a) ![]()
b) 
c) 
2. (10 points) Examine the pairs of compounds below and identify their relationship to one another. Use the letter codes below:
A - cis-trans isomers
B - conformational isomers
C - resonance forms
D - identical structures
E - none of the above
a) ![]()
b) 
c) ![]()
d) 
e) 
3. (10 points) Same as the previous problem set:
A - cis-trans isomers
B - conformational isomers
C - resonance forms
D - identical structures
E - none of the above
a) 
b) 
c) 
d) 
e) 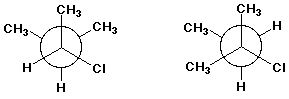
4. (10 points) For the compound shown below:
![]()
Write an accurate three-dimensional structure that shows all bonds and their proper orientation.

Write an accurate line structure.
![]()
5. (10 points) The pKa values for ethanol (CH3CH2OH) and ammonium ion (NH4+) are 16 and 10, respectively.
Using these compounds and their conjugate bases, write a balanced acid-base equilibrium reaction that would be favorable as written to the right.
6. (15 points) Shown below are incomplete structures. Add the missing substituents to show the proper structure appropriate for the name.
To help you keep organized, the preferred location of the carbon with the methyl substituent is always located with a dot.
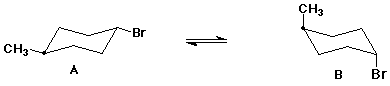
trans-1-bromo-4-methylcyclohexane (both chair conformations)
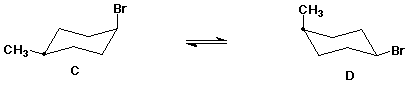
cis-1-bromo-4-methylcyclohexane (both chair conformations)
The structure shown below should correspond to one of your completed structures above.
Is it A, B, C, or D?

Write the corresponding Newman diagram for its other chair conformation.
7. (20 points) The addition of HCl to ethene occurs in a two-step mechanism.
Clearly write each of the two steps, including all of the following for each step:
all the bonds in the reactants and the products
the specific bonds that are broken and made
identification of nucleophiles and electrophiles
electron-pushing arrows that show the electron flow
a likely transition state structure
Step 1:

Step 2:

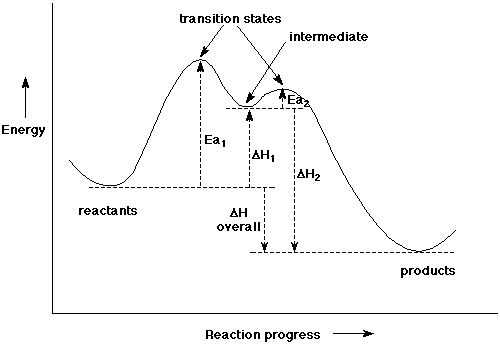
8. (10 points) Write an approximate reaction energy diagram for the two-step mechanism you wrote in the previous problem. Clearly indicate all of the following on your diagram:
the axis labels
the reactants and products
the transition states
the intermediate
activation energy for each step
H for each step
overall Ea and H
Use the following values for energies (in kcal/mole):
Ea1 = 25, Ea2 = 2, H1 = +20, H2 = -30